Abstract
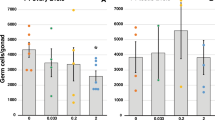
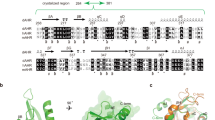
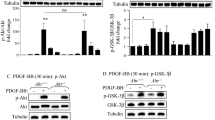
Polycyclic aromatic hydrocarbons (PAHs) are toxic chemicals released into the environment by fossil fuel combustion. Moreover, a primary route of human exposure to PAHs is tobacco smoke1,2. Oocyte destruction and ovarian failure occur in PAH-treated mice1,2, and cigarette smoking causes early menopause in women1,3. In many cells, PAHs activate the aromatic hydrocarbon receptor (Ahr), a member of the Per-Arnt-Sim family of transcription factors4,5. The Ahr is also activated by dioxin, one of the most intensively studied environmental contaminants. Here we show that an exposure of mice to PAHs induces the expression of Bax in oocytes6, followed by apoptosis. Ovarian damage caused by PAHs is prevented by Ahr or Bax inactivation. Oocytes microinjected with a Bax promoter–reporter construct show Ahr-dependent transcriptional activation after PAH, but not dioxin, treatment, consistent with findings that dioxin is not cytotoxic to oocytes. This difference in the action of PAHs versus dioxin is conveyed by a single base pair flanking each Ahr response element in the Bax promoter. Oocytes in human ovarian biopsies grafted into immunodeficient mice also accumulate Bax and undergo apoptosis after PAH exposure in vivo. Thus, Ahr-driven Bax transcription is a novel and evolutionarily conserved cell-death signaling pathway responsible for environmental toxicant-induced ovarian failure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Mattison, D.R. et al. The effect of smoking on oogenesis, fertilization and implantation. Sem. Reprod. Health 7, 291–304 (1989).
Sussman, N.B., Mazumdar, S. & Mattison, D.R. Modeling adverse environmental impacts on the reproductive system. J. Women's Health 8, 217–226 (1999).
Jick, H. & Porter, J. Relation between smoking and age of natural menopause. Report from the Boston Collaborative Drug Surveillance Program, Boston University Medical Center. Lancet 1, 1354–1355 (1977).
Hankinson, O. The aryl hydrocarbon receptor complex. Ann. Rev. Pharmacol. Toxicol. 35, 307–340 (1995).
Wilson, C.L. & Safe, S. Mechanisms of ligand-induced aryl hydrocarbon receptor-mediated biochemical and toxic responses. Toxicol. Pathol. 26, 657–671 (1998).
Oltvai, Z.N., Milliman, C.L. & Korsmeyer, S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74, 609–619 (1993).
Morita, Y. & Tilly, J.L. Oocyte apoptosis: like sand through an hourglass. Dev. Biol. 213, 1–17 (1999).
Perez, G.I., Knudson, C.M., Leykin, L., Korsmeyer, S.J. & Tilly, J.L. Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nature Med. 3, 1228–1232 (1997).
Perez, G.I. et al. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nature Genet. 21, 200–203 (1999).
Morita, Y. et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nature Med. 6, 1109–1114 (2000).
Pru, J.K. & Tilly, J.L. Programmed cell death in the ovary: insights and future prospects using genetic technologies. Mol. Endocrinol. 15, 845–853 (2001).
Robles, R. et al. The aryl hydrocarbon receptor, a basic helix-loop-helix transcription factor of the PAS gene family, is required for normal ovarian germ cell dynamics in the mouse. Endocrinology 141, 450–453 (2000).
Mattison, D.R. & Nightingale, M.R. The biochemical and genetic characteristics of murine ovarian aryl hydrocarbon (benzo[a]pyrene) hydroxylase activity and its relationship to primordial oocyte destruction by polycyclic aromatic hydrocarbons. Toxicol. Appl. Pharmacol. 56, 399–408 (1980).
Heidel, S.M., Czuprynski, C.J. & Jefcoate, C.R. Bone marrow stromal cells constitutively express high levels of cytochrome P4501B1 that metabolize 7,12-dimethylbenz[a]anthracene. Mol. Pharmacol. 54, 1000–1006 (1998).
Blank, J.A., Tucker, A.N., Sweatlock, J., Gasiewicz, T.A. & Luster, M.I. α-Naphthoflavone antagonism of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced murine lymphocyte ethoxyresorufin-O-deethylase activity and immunosuppression. Mol. Pharmacol. 32, 169–172 (1987).
Mann, K.K. et al. The role of polycyclic aromatic hydrocarbon metabolism in dimethylbenz[a]anthracene-induced pre-B lymphocyte apoptosis. Toxicol. Appl. Pharmacol. 161, 10–22 (1999).
Silbergeld, E.K. & Mattison, D.R. Experimental and clinical studies on the reproductive toxicology of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Am. J. Ind. Med. 11, 131–144 (1987).
Shen, E.S. & Whitlock J.P. Jr. Protein-DNA interactions at a dioxin-responsive enhancer. Mutational analysis of the DNA-binding site for the liganded Ah receptor. J. Biol. Chem. 267, 6815–6819 (1992).
Lusska, A., Shen, E. & Whitlock J. P. Jr. Protein-DNA interactions at a dioxin-responsive enhancer. Analysis of six bona fide DNA-binding sites for the liganded Ah receptor. J. Biol. Chem. 268, 6575–6580 (1993).
Knudson, C.M., Tung, K.S., Tourtellotte, W.G., Brown, G.A. & Korsmeyer, S.J. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 270, 96–99 (1995).
Eppig, J.J. & O'Brien, M.J. Development in vitro of mouse oocytes from primordial follicles. Biol. Reprod. 54, 197–207 (1996).
Morita, Y., Perez, G.I., Maravei, D.V., Tilly, K.I. & Tilly, J.L. Targeted expression of Bcl-2 in mouse oocytes inhibits ovarian follicle atresia and prevents spontaneous and chemotherapy-induced oocyte apoptosis in vitro. Mol. Endocrinol. 13, 841–850 (1999).
Tilly, J.L., Tilly, K.I., Kenton, M.L. & Johnson, A.L. Expression of members of the bcl-2 gene family in the immature rat ovary: equine chorionic gonadotropin-mediated inhibition of granulosa cell apoptosis is associated with decreased bax and constitutive bcl-2 and bcl-xlong messenger RNA levels. Endocrinology 136, 232–241 (1995).
Pru, J. K. et al. Interferon-tau suppresses prostaglandin-F2α secretion independently of the mitogen-activated protein kinase and nuclear factor-κB pathways. Biol. Reprod. 64, 965–973 (2001).
Shi, S.R., Cote, R.J. & Taylor, C.R. Antigen retrieval immunohistochemistry: past, present, and future. J. Histochem. Cytochem. 45, 327–343 (1991).
Jurisicova, A., Latham, K.E., Casper, R.F. & Varmuza, S.L. Expression and regulation of genes associated with cell death during murine preimplantation embryo development. Mol. Reprod. Dev. 51, 243–253 (1998).
Igata, E. et al. Molecular cloning and functional analysis of the murine Bax gene promoter. Gene 238, 407–415 (1999).
Qvist, R., Blackwell, L.F., Bourne, H. & Brown, J.B. Development of mouse ovarian follicles from primary to preovulatory stages in vitro. J. Reprod. Fertil. 89, 169–180 (1990).
Weissman, A. et al. Preliminary experience with subcutaneous human ovarian cortex transplantation in the NOD-SCID mouse. Biol. Reprod. 60, 1462–1467 (1999).
Mattison, D.R. & Thorgeirsson, S.S. Ovarian aryl hydrocarbon hydroxylase activity and primordial oocyte toxicity of polycyclic aromatic hydrocarbons in mice. Cancer Res. 39, 3471–3475 (1979).
Acknowledgements
We thank K.I. Tilly, X.-J. Tao and T. Manganaro for technical help, S. Riley for assistance with the image analysis, and I. Schiff for critical reading of the manuscript. This study was supported by the National Institutes of Health (R01-ES08430), the Canadian Toxic Substances Research Initiative, the Harvard Center of Excellence in Women's Health, and Vincent Memorial Research Funds. We conducted this work while T.M. and J.L. were Research Fellows supported by the Finnish Foundation for Pediatric Research and the Finnish Cultural Foundation, A.J. was a Research Fellow supported by the Canadian Institutes of Health Research, and J.K.P. was a Research Fellow supported by the Lalor Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matikainen, T., Perez, G., Jurisicova, A. et al. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat Genet 28, 355–360 (2001). https://doi.org/10.1038/ng575
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng575
This article is cited by
-
Prenatal exposure to benzo[a]pyrene depletes ovarian reserve and masculinizes embryonic ovarian germ cell transcriptome transgenerationally
Scientific Reports (2023)
-
Premature ovarian insufficiency associated with environmental chemical exposure among Korean women: a study based on the Korean National Environmental Health Survey (2009–2012)
Molecular & Cellular Toxicology (2023)
-
Association of the urinary polycyclic aromatic hydrocarbons with sex hormones stratified by menopausal status older than 20 years: a mixture analysis
Environmental Science and Pollution Research (2023)
-
Pharmacological blockage of the AHR-CYP1A1 axis: a call for in vivo evidence
Journal of Molecular Medicine (2022)
-
Discovery and Mechanistic Characterization of a Select Modulator of AhR-regulated Transcription (SMAhRT) with Anti-cancer Effects
Apoptosis (2021)