Abstract
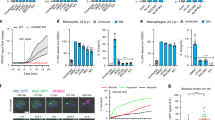
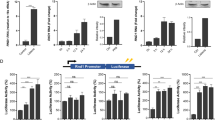
Vertebrate immunity to infection enlists a newly identified family of 47-kilodalton immunity-related GTPases (IRGs). One IRG in particular, Irgm1, is essential for macrophage host defense against phagosomal pathogens, including Mycobacterium tuberculosis (Mtb). Here we show that Irgm1 targets the mycobacterial phagosome through lipid-mediated interactions with phosphatidylinositol-3,4-bisphosphate (PtdIns(3,4)P2) and PtdIns(3,4,5)P3. An isolated Irgm1 amphipathic helix conferred lipid binding in vitro and in vivo. Substitutions in this region blocked phagosome recruitment and failed to complement the antimicrobial defect in Irgm1−/− macrophages. Removal of PtdIns(3,4,5)P3 or inhibition of class I phosphatidylinositol-3-OH kinase (PI(3)K) mimicked this effect in wild-type cells. Cooperation between Irgm1 and PI(3)K further facilitated the engagement of Irgm1 with its fusogenic effectors at the site of infection, thereby ensuring pathogen-directed responses during innate immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Hingley-Wilson, S.M., Sambandamurthy, V.K. & Jacobs, W.R. Jr. Survival perspectives from the world's most successful pathogen, Mycobacterium tuberculosis. Nat. Immunol. 4, 949–955 (2003).
Pieters, J. Mycobacterium tuberculosis and the macrophage. Maintaining a balance. Cell Host Microbe 3, 399–407 (2008).
MacMicking, J.D., Taylor, G.A. & McKinney, J.D. Immune control of tuberculosis by IFN-γ-inducible LRG-47. Science 302, 654–659 (2003).
Gutierrez, M.G. et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119, 753–766 (2004).
Singh, S.B., Davis, A.S., Taylor, G.A. & Deretic, V. Human IRGM induces autophagy to eliminate intracellular bacteria. Science 313, 1438–1441 (2006).
Xu, Y. et al. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity 27, 135–144 (2007).
Feng, C.G. et al. Mice deficient in LRG-47 display increased susceptibility to mycobacterial infection associated with the induction of lymphopenia. J. Immunol. 172, 1163–1168 (2004).
MacMicking, J.D. et al. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA 94, 5243–5248 (1997).
Ng, V.H., Cox, J.S., Sousa, A.O., MacMicking, J.D. & McKinney, J.D. Role of KatG catalase-peroxidase in mycobacterial pathogenesis: Countering the oxidative burst. Mol. Microbiol. 52, 1291–1302 (2004).
Malik, S. et al. Alleles of the NRAMP1 gene are risk factors for pediatric tuberculosis disease. Proc. Natl. Acad. Sci. USA 102, 12183–12188 (2005).
Bekpen, C. et al. The interferon-inducible p47 (IRG) GTPases in vertebrates: loss of the cell autonomous resistance mechanism in the human lineage. Genome Biol. 6, R92 (2005).
Shenoy, A.R. et al. Emerging themes in IFN-γ-induced macrophage immunity by the p47 and p65 GTPase families. Immunobiology 8, 771–784 (2008).
Taylor, G.A., Feng, C.G. & Sher, A. p47 GTPases: regulators of immunity to intracellular pathogens. Nat. Rev. Immunol. 4, 100–109 (2004).
MacMicking, J.D. IFN-inducible GTPases and immunity to intracellular pathogens. Trends Immunol. 25, 601–609 (2004).
Stephens, D.J. & Banting, G. Specificity of interaction between adaptor-complex medium chains and the tyrosine-based sorting motifs of TGN38 and lgp120. Biochem. J. 335, 567–572 (1998).
Martens, S. et al. Mechanisms regulating the positioning of mouse p47 resistance GTPases LRG-47 and IIGP1 on cellular membranes: retargeting to plasma membrane induced by phagocytosis. J. Immunol. 173, 2594–2606 (2004).
Ghosh, A., Uthaiah, R., Howard, J., Herrmann, C. & Wolf, E. Crystal structure of IIGP1: a paradigm for interferon-inducible p47 resistance GTPases. Mol. Cell 10, 727–739 (2004).
McLaughlin, S. & Murray, D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature 438, 605–611 (2005).
Manna, D., Albanese, A., Park, W.S. & Cho, W. Mechanistic basis of differential cellular responses of phosphatidylinositol 3,4-bisphosphate- and phosphatidylinositol 3,4,5-trisphosphate-binding pleckstrin homology domains. J. Biol. Chem. 282, 32093–32105 (2007).
Chua, J. & Deretic, V. Mycobacterium tuberculosis reprograms waves of phosphatidylinositol 3-phosphate on phagosomal organelles. J. Biol. Chem. 279, 36983–36992 (2004).
Fischer, K. et al. Mycobacterial lysocardiolipin is exported from phagosomes upon cleavage of cardiolipin by a macrophage-derived lysosomal phospholipase A2. J. Immunol. 167, 2187–2192 (2001).
Koyasu, S. The role of PI(3)K in immune cells. Nat. Immunol. 4, 313–319 (2003).
Kamen, L.A., Levinsohn, J. & Swanson, J.A. Differential association of phosphatidylinositol 3-kinase, SHIP-1, and PTEN with forming phagosomes. Mol. Biol. Cell 18, 2463–2472 (2007).
Kamen, L.A., Levinsohn, J., Cadwallader, A., Tridandapani, S. & Swanson, J.A. SHIP-1 increases early oxidative burst and regulates phagosome maturation in macrophages. J. Immunol. 180, 7497–7505 (2008).
Heo, W.D. et al. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science 314, 1458–1461 (2006).
Vieira, O. et al. Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J. Cell Biol. 155, 19–25 (2001).
Vanhaesebroeck, B. et al. Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 70, 535–602 (2001).
Hayakawa, M. et al. Synthesis and biological evaluation of 4-morpholino-2-phenylquinazolines and related derivatives as novel PI3 kinase p110alpha inhibitors. Bioorg. Med. Chem. 14, 6847–6858 (2006).
Jackson, S.P. et al. PI3-kinase p110β: a new target for antithrombotic therapy. Nat. Med. 11, 507–514 (2005).
Condliffe, A.M. et al. Sequential activation of class IB and class 1A PI(3)K is important for the primed respiratory burst of human but not murine neutrophils. Blood 106, 1432–1440 (2005).
Rodriguez-Viciana, P. et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 370, 527–532 (1994).
Chamberlain, M.D., Berry, T.R., Pastor, M.C. & Anderson, D.H. The p85alpha subunit of phosphatidylinositol 3′-kinase binds to and stimulates the GTPase activity of Rab proteins. J. Biol. Chem. 279, 48607–48614 (2004).
Bos, J.L., Rehmann, H. & Wittinghofer, A. GEFs and GAPs: critical elements in the control of small G proteins. Cell 129, 865–877 (2007).
Buxton, P. et al. Identification and characterization of Snapin as a ubiquitously expressed SNARE-binding protein that interacts with SNAP23 in non-neuronal cells. Biochem. J. 15, 433–440 (2003).
Pan, P.Y., Tian, J.H. & Sheng, Z.H. Snapin facilitates the synchronization of synaptic vesicle fusion. Neuron 61, 412–424 (2009).
Lu, L., Cai, Q., Tian, J.H. & Sheng, Z.H. Snapin associates with late endocytic compartments and interacts with late endosomal SNAREs. Biosci. Rep. 29, 261–269 (2009).
Stamnes, M.A. et al. An integral membrane component of coatomer-coated transport vesicles defines a family of proteins involved in budding. Proc. Natl. Acad. Sci. USA 92, 8011–8015 (1995).
Hawkins, P.T., Anderson, K.E., Davidson, K. & Stephens, L.R. Signaling through class I PI(3)Ks in mammalian cells. Biochem. Soc. Trans. 34, 647–662 (2006).
Deb, D.K. et al. Activation of protein kinase Cδ by IFN-γ. J. Immunol. 171, 267–273 (2003).
Yeung, T. et al. Receptor activation alters inner surface potential during phagocytosis. Science 313, 347–351 (2006).
Ferguson, K.M. et al. Structural basis for the discrimination of 3-phosphoinositides by pleckstrin homology domains. Mol. Cell 6, 373–384 (2000).
Rodriguez-Viciana, P. et al. Role of phosphoinositide 3-OH kinase in cell transformation and control of actin cytoskeleton by Ras. Cell 89, 457–467 (1997).
Christoforidis, S. et al. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat. Cell Biol. 1, 249–252 (1999).
Zhao, Z. et al. Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe 4, 458–469 (2008).
Jayachandran, R. et al. Survival of mycobacteria in macrophages is mediated by coronin 1-dependent activation of calcineurin. Cell 130, 37–50 (2007).
Kuijl, C. et al. Intracellular bacterial growth is controlled by a kinase network around PKB/AKT1. Nature 450, 725–730 (2007).
Feng, C.G. et al. The immunity-related GTPase Irgm1 promotes the expansion of activated CD4+ T cell populations by preventing interferon-γ-induced cell death. Nat. Immunol. 9, 1279–1287 (2008).
Luo, Y., Szilvasi, A., Chen, X., DeWolf, W.C. & O'Donnell, M.A. A novel method for monitoring Mycobacterium bovis BCG trafficking with recombinant BCG expressing green fluorescent protein. Clin. Diagn. Lab. Immunol. 3, 761–768 (1996).
Taguchi, T., Pypaert, M. & Warren, G. Biochemical sub-fractionation of the mammalian Golgi apparatus. Traffic 4, 344–352 (2003).
Mileykovskaya, E. et al. Cardiolipin binds nonyl acridine orange by aggregating the dye at exposed hydrophobic domains on bilayer surfaces. FEBS Lett. 507, 187–190 (2001).
Acknowledgements
We thank L. Cantley (Harvard Medical School), P. De Camilli (Yale University School of Medicine), J. Galan (Yale University School of Medicine), P. Lengyel (Yale University School of Medicine), R. Lin (Stony Brook University), J. Lucocq (University of Dundee), T. Meyer (Stanford University), C. Roy (Yale University School of Medicine), B. Vanhaesebroeck (University of London) and G. Warren (Vienna Biocenter) for antibodies, plasmids or cDNAs used in this study; P. Cresswell (Yale University School of Medicine) for the yeast two-hybrid mouse embryonic fibroblast library; G. Taylor (Duke University Medical Center) for Irgm1−/− mice, Y. Lu (University of Iowa) for BCG-GFP; and A. Shenoy for help with Irgm1 crystallographic modeling. Supported by the National Institute of Allergy and Infectious Diseases of the US National Institutes of Health (R01 AI068041-01A1), the Burroughs-Wellcome Fund (1007845), Edward R. Mallinckrodt Foundation (R06152), the Searle Foundation (05-F-114), the Cancer Research Institute, the W.W. Winchester Foundation (J.D.M.) and the Japanese Society for the Promotion of Science (T.M.).
Author information
Authors and Affiliations
Contributions
S.T., T.M., H.-P.C., M.P. and J.D.M. did the experimental design, data analysis and interpretation; S.T., H.-P.C. and T.M. did molecular and biochemical analyses (affinity purification, gel filtration, yeast-two hybrid, in vitro assays); S.T., T.M., H.-P.C. and J.D.M. did scanning confocal microscopy; M.P. and J.D.M. did transmission electron microscopy of immunolabeled cryosections; J.D.M. wrote the manuscript; and all authors discussed the results and commented on the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Tables 1–2 (PDF 6130 kb)
Supplementary Movie 1
Live imaging of Irgm1 (RFP-Irgm1, pseudocolored green) translocation to sites of M. bovis BCG internalization (white arrows) in IFN-γ-activated RAW264.7 macrophages. EGFP-BCG, pseudocolored red. (AVI 11535 kb)
Supplementary Movie 2
Triple-labeled live imaging of Irgm1 translocation to PtdIns(3,4,5)P3-pseudopods internalizing M. bovis BCG in IFN-γ-activated RAW264.7 cells (white arrow). CFP-Irgm1 (pseudocolored green); YFP-GRP1-PH (pseudocolored red); Cy5-BCG (blue). (AVI 951 kb)
Rights and permissions
About this article
Cite this article
Tiwari, S., Choi, HP., Matsuzawa, T. et al. Targeting of the GTPase Irgm1 to the phagosomal membrane via PtdIns(3,4)P2 and PtdIns(3,4,5)P3 promotes immunity to mycobacteria. Nat Immunol 10, 907–917 (2009). https://doi.org/10.1038/ni.1759
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1759
This article is cited by
-
Mitochondria in the Pathogenesis of Systemic Lupus Erythematosus
Current Rheumatology Reports (2022)
-
Macrophage-microbe interaction: lessons learned from the pathogen Mycobacterium tuberculosis
Seminars in Immunopathology (2018)
-
Loss of the interferon-γ-inducible regulatory immunity-related GTPase (IRG), Irgm1, causes activation of effector IRG proteins on lysosomes, damaging lysosomal function and predicting the dramatic susceptibility of Irgm1-deficient mice to infection
BMC Biology (2016)
-
Mice lack of LRG-47 display the attenuated outcome of infection with Schistosoma japonicum
Parasitology Research (2016)
-
IRGM1 enhances B16 melanoma cell metastasis through PI3K-Rac1 mediated epithelial mesenchymal transition
Scientific Reports (2015)