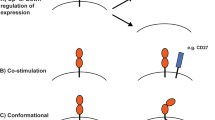
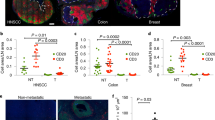
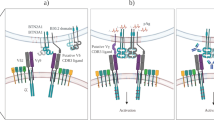
Abstract
T cells bearing γδ T cell antigen receptors (TCRs) function in lymphoid stress surveillance. However, the contribution of γδ TCRs to such responses is unclear. Here we found that the TCR of a human Vγ4Vδ5 clone directly bound endothelial protein C receptor (EPCR), which allowed γδ T cells to recognize both endothelial cells targeted by cytomegalovirus and epithelial tumors. EPCR is a major histocompatibility complex–like molecule that binds lipids analogously to the antigen-presenting molecule CD1d. However, the Vγ4Vδ5 TCR bound EPCR independently of lipids, in an antibody-like way. Moreover, the recognition of target cells by γδ T cells required a multimolecular stress signature composed of EPCR and costimulatory ligand(s). Our results demonstrate how a γδ TCR mediates recognition of broadly stressed human cells by engaging a stress-regulated self antigen.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Girardi, M. et al. Regulation of cutaneous malignancy by γδ T cells. Science 294, 605–609 (2001).
Born, W. et al. Immunoregulatory functions of γδ T cells. Adv. Immunol. 71, 77–144 (1999).
Ma, Y. et al. Contribution of IL-17a producing γδ T cells to the efficacy of anticancer chemotherapy. J. Exp. Med. 208, 491–503 (2011).
Wilhelm, M. γδ T cells for immune therapy of patients with lymphoid malignancies. Blood 102, 200–206 (2003).
Costa, G. et al. Control of Plasmodium falciparum erythrocytic cycle: γδ T cells target the red blood cell-invasive merozoites. Blood 118, 6952–6962 (2011).
Peng, G. et al. Tumor-infiltrating γδ T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity 27, 334–348 (2007).
Brandes, M., Willimann, K. & Moser, B. Professional antigen-presentation function by human γδ T Cells. Science 309, 264–268 (2005).
Toulon, A. et al. A role for human skin-resident T cells in wound healing. J. Exp. Med. 206, 743–750 (2009).
Dieli, F. et al. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 67, 7450–7457 (2007).
Zarski, J. et al. Repeated administrations of IPH1101 in monotherapy or combined with low dose IL2 (2 M IU) in patients chronically infected with hepatitis C virus: efficacy, safety and immunomonitoring results from a phase 2 study. Hepatology 50, 1031A (2009).
Bonneville, M., O'Brien, R.L. & Born, W.K. γδ T cell effector functions: a blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 10, 467–478 (2010).
Hayday, A.C. γδ T cells and the lymphoid stress-surveillance response. Immunity 31, 184–196 (2009).
Strid, J. et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat. Immunol. 9, 146–154 (2008).
Adams, E.J., Chien, Y.H. & Garcia, K.C. Structure of a γδ T cell receptor in complex with the nonclassical MHC T22. Science 308, 227–231 (2005).
Scotet, E. et al. Tumor recognition following Vγ9Vδ2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity 22, 71–80 (2005).
Bukowski, J.F., Morita, C.T. & Brenner, M.B. Recognition and destruction of virus-infected cells by human γδ CTL. J. Immunol. 153, 5133–5140 (1994).
Constant, P. et al. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science 264, 267–270 (1994).
Gober, H.J. et al. Human T cell receptor γδ cells recognize endogenous mevalonate metabolites in tumor cells. J. Exp. Med. 197, 163–168 (2003).
Halary, F. et al. Shared reactivity of Vdelta2-neg γδ T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J. Exp. Med. 201, 1567–1578 (2005).
Maeurer, M., Zitvogel, L., Elder, E., Storkus, W.J. & Lotze, M.T. Human intestinal Vδ1+ T cells obtained from patients with colon cancer respond exclusively to SEB but not to SEA. Natural Immunity 14, 188–197 (1995).
Spada, F.M. et al. Self-Recognition of CD1 by γδ T cells: implications for innate immunity. J. Exp. Med. 191, 937–948 (2000).
Groh, V., Steinle, A., Bauer, S. & Spies, T. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science 279, 1737–1740 (1998).
Déchanet, J. et al. Implication of γδ T cells in the human immune response to cytomegalovirus. J. Clin. Invest. 103, 1437–1449 (1999).
Pitard, V. et al. Long-term expansion of effector-memory Vδ2− γδ T cells is a specific blood signature of CMV infection. Blood 112, 1317–1324 (2008).
Ehl, S. et al. A variant of SCID with specific immune responses and predominance of γδ T cells. J. Clin. Invest. 115, 3140–3148 (2005).
Vermijlen, D. et al. Human cytomegalovirus elicits fetal γδ T cell responses in utero. J. Exp. Med. 207, 807–821 (2010).
Knight, A. et al. The role of Vδ2-negative γδ T cells during cytomegalovirus reactivation in recipients of allogeneic stem cell transplantation. Blood 116, 2164–2172 (2010).
Couzi, L. et al. Cytomegalovirus-induced γδ T cells associate with reduced cancer risk after kidney transplantation. J. Am. Soc. Nephrol. 21, 181–188 (2010).
Devaud, C. et al. Antitumor activity of γδ T cells reactive against cytomegalovirus-infected cells in a mouse xenograft tumor model. Cancer Res. 69, 3971–3978 (2009).
Couzi, L. et al. Common features of γδ T cells and CD8+ αβ T cells responding to human cytomegalovirus infection in kidney transplant recipients. J. Infect. Dis. 200, 1415–1424 (2009).
Lafarge, X. et al. Expression of MHC class I receptors confers functional intraclonal heterogeneity to a reactive expansion of γδ T cells. Eur. J. Immunol. 35, 1896–1905 (2005).
Oganesyan, V. et al. The crystal structure of the endothelial protein C receptor and a bound phospholipid. J. Biol. Chem. 277, 24851–24854 (2002).
Esmon, C.T. Structure and functions of the endothelial cell protein C receptor. Crit. Care Med. 32, S298–S301 (2004).
Scheffer, G.L. et al. Expression of the vascular endothelial cell protein C receptor in epithelial tumor cells. Eur. J. Cancer 38, 1535–1542 (2002).
Tsuneyoshi, N. et al. Expression and anticoagulant function of the endothelial cell protein C receptor (EPCR) in cancer cell lines. Thromb. Haemost. 85, 356–361 (2001).
Cheng, T. et al. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat. Med. 9, 338–342 (2003).
Schild, H. et al. The nature of MHC recognition by γδ T cells. Cell 76, 29–37 (1994).
Tikhonova, A.N. et al. αβ T cell receptors that do not undergo major histocompatibility complex-specific thymic selection possess antibody-like recognition specificities. Immunity 36, 79–91 (2012).
Noriega, V., Redmann, V., Gardner, T. & Tortorella, D. Diverse immune evasion strategies by human cytomegalovirus. Immunologic Res 1–12 (2012).
Garrido, F., Cabrera, T. & Aptsiauri, N. “Hard” and “soft” lesions underlying the HLA class I alterations in cancer cells: Implications for immunotherapy. Int. J. Cancer 127, 249–256 (2010).
Chodaczek, G., Papanna, V., Zal, M.A. & Zal, T. Body-barrier surveillance by epidermal γδ TCRs. Nat. Immunol. 13, 272–282 (2012).
Squizzato, A., Gerdes, V.E. & Buller, H.R. Effects of human cytomegalovirus infection on the coagulation system. Thromb. Haemost. 93, 403–410 (2005).
Couzi, L. et al. Antibody-dependent anti-cytomegalovirus activity of human γδ T cells expressing CD16 (FcγRIIIa). Blood 119, 1418–1427 (2012).
Géronimi, F. et al. Highly efficient lentiviral gene transfer in CD34+ and CD34+38−lin− cells from mobilized peripheral blood after cytokine prestimulation. Stem Cells 21, 472–480 (2003).
Peterman, S.M., Dufresne, C.P. & Horning, S. The use of a hybrid linear trap-FT-ICR mass spectrometer for on-line high resolution-high mass accuracy bottom-up sequencing. J. Biomol. Tech. 16, 112–124 (2005).
Eng, J.K., McCormack, A.L. & Yates Iii, J.R. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 5, 976–989 (1994).
Willcox, B.E. et al. TCR binding to peptide-MHC stabilizes a flexible recognition interface. Immunity 10, 357–365 (1999).
Acknowledgements
We thank M.-L. Michel, M. Swamy and F. Mohammed for contributions to this project; D. Price for discussions; and V. Venturi for analysis of TCR nontemplated nucleotide additions. Supported by Fondation pour la Recherche Médicale (DEQ20051205738 and DEQ20110421287), Agence Nationale de la Recherche (ANR-05-JCJC-0129-01), Ligue Nationale contre le Cancer, Association pour la Recherche sur le Cancer (A09-1-5022), Institut National contre le Cancer (INCa TUMOSTRESS) Cancer Research UK (C17422-A7986 and C17422-A11740 to B.E.W., supporting C.R.W. and M.S.), the Wellcome Trust (T.S. and A.C.H.) and Boehringer Ingelheim Fonds (T.S. and A.C.H.).
Author information
Authors and Affiliations
Contributions
C.R.W. and V.P. did most of the experiments, analyzed data and designed experiments; S.N., L.C. and M.S. did experiments and analyzed data; T.S. did gene profiling and analysis; J.-F.M. helped supervise research; and A.C.H., B.E.W., J.D.-M. analyzed and interpreted data, designed and supervised research and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 391 kb)
Supplementary Table 1
Illumina expression profiling of 2E9L+ vs 2E9L(−) cell lines (XLS 48 kb)
Rights and permissions
About this article
Cite this article
Willcox, C., Pitard, V., Netzer, S. et al. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat Immunol 13, 872–879 (2012). https://doi.org/10.1038/ni.2394
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2394
This article is cited by
-
Structural vulnerability in EPCR suggests functional modulation
Scientific Reports (2024)
-
Local γδ T cells: translating promise to practice in cancer immunotherapy
British Journal of Cancer (2023)
-
γδ T cells: origin and fate, subsets, diseases and immunotherapy
Signal Transduction and Targeted Therapy (2023)
-
Identification of a broad lipid repertoire associated to the endothelial cell protein C receptor (EPCR)
Scientific Reports (2022)
-
Unconventional T cells and kidney disease
Nature Reviews Nephrology (2021)