Abstract
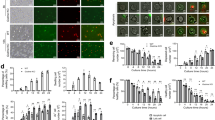
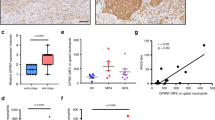
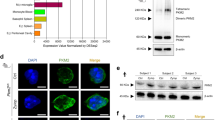
In neutrophils, superoxide anion production generally accompanies chemotaxis and functions in killing invading pathogens. The GIT2 GTPase-activating protein binds to the guanine nucleotide–exchange factor αPIX. Here we show that GIT2 was necessary for directional chemotaxis and for the suppression of superoxide production in G protein–coupled receptor–stimulated neutrophils. GIT2 was also necessary for the orientation of superoxide production toward chemoattractant sources. GIT2 suppressed the activity of ADP ribosylation factor 1 and was a component of the Gβγ subunit–mediated direction-sensing machinery 'downstream' of G protein–coupled receptor signaling. This study establishes a function for GIT2 in linking chemotaxis and superoxide production in neutrophils and shows that loss of GIT2 in vivo leads to an immunodeficient state.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
02 June 2006
In the version of this article initially published online, the word "in" is missing on page 3, column 2, line 21; this should read "normal phosphorylation of PAK1 in Git2+/− neutrophils (Fig. 4d)." Also, in the supplementary information, the Greek mu symbol is missing from the units. The errors have been corrected for all versions of the article.
References
Cross, A.R. & Segel, A.W. The NADPH oxidase of professional phagocytes: prototype of the NOX electron transport chain systems. Biochim. Biophys. Acta 1657, 1–22 (2004).
Murphy, P.M. The molecular biology of leukocyte chemoattractant receptors. Annu. Rev. Immunol. 12, 593–633 (1994).
Li, Z. et al. Directional sensing requires Gβγ-mediated PAK1 and PIXα-dependent activation of Cdc42. Cell 114, 215–227 (2003).
Bokoch, G.M. Biology of the p21-activated kinase. Annu. Rev. Biochem. 72, 743–781 (2003).
Dana, R.R., Eigsti, C., Holmes, K.L. & Leto, T. A regulatory role for ADP-ribosylation factor 6 (ARF6) in activation of the phagocyte NADPH oxidase. J. Biol. Chem. 275, 32566–32571 (2000).
Káldi, K. et al. Contribution of phopholipase D and a brefeldin A-sensitive ARF to chemoattractant-induced superoxide production and secretion of human neutrophils. J. Leukoc. Biol. 71, 695–700 (2002).
Bagrodia, S. et al. A tyrosine-phosphorylated protein that binds to an important regulatory region on the cool family of p21-activated kinase-binding proteins. J. Biol. Chem. 274, 22393–22400 (1999).
Premont, R.T., Claing, A., Vitale, N., Perry, S.J. & Lefkowits, R.J. The GIT family of ADP-ribosylation factor GTPase-activating proteins. Functional diversity of GIT2 through alternative splicing. J. Biol. Chem. 275, 22373–22380 (2000).
Schmalzigaug, R. & Premont, R. in ARF Family GTPases 159–183 (Kluwer Academic, Dordrecht, 2003).
Zhao, Z.S., Manser, E., Loo, T.H. & Lim, L. Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol. Cell. Biol. 20, 6354–6363 (2000).
Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 4, 1–23 (2004).
Boyden, S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J. Exp. Med. 115, 453–466 (1962).
Hirsch, E. et al. Central role for G protein-coupled phosphoinositide 3-kinase γ in inflammation. Science 287, 1049–1053 (2000).
Li, Z. et al. Roles of PLC-β2 and -β3 and PI3Kγ in chemoattractant-mediated signal transduction. Science 287, 1046–1049 (2000).
Sasaki, T. et al. Function of PI3Kγ in thymocyte development, T cell activation, and neutrophil migration. Science 287, 1040–1046 (2000).
Zigmond, S.H. Orientation chamber in chemotaxis. Methods Enzymol. 162, 65–72 (1988).
Phee, H., Abraham, R.T. & Weiss, A. Dynamic recruitment of PAK1 to the immunological synapse is mediated by PIX independently of SLP-76 and Vav1. Nat. Immunol. 6, 608–617 (2005).
Weiner, O.D. et al. A PtdInsP3- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat. Cell Biol. 4, 509–513 (2002).
Wang, F. et al. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat. Cell Biol. 4, 513–518 (2002).
Hannigan, M. et al. Neutrophils lacking phosphoinositide 3-kinase γ show loss of directionality during N-formyl-Met-Leu-Phe-induced chemotaxis. Proc. Natl. Acad. Sci. USA 99, 3603–3608 (2002).
Brazil, D.P., Yang, Z.Z. & Hemmings, B.A. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem. Sci. 29, 233–242 (2004).
Dekker, L.V. & Segal, A.W. Signals to move cells. Science 287, 982–985 (2000).
Brock, C. et al. Roles of Gβγ in membrane recruitment and activation of p110γ/p101 phosphoinositide 3-kinase γ. J. Cell Biol. 160, 89–99 (2003).
Chardin, P. & McCormick, F. Brefeldin A: the advantage of being uncompetitive. Cell 97, 153–155 (1999).
Mazaki, Y. et al. An ADP-ribosylation factor GTPase-activating protein Git2-short/KIAA0148 is involved in subcellular localization of paxillin and actin cytoskeletal organization. Mol. Biol. Cell 12, 645–662 (2001).
Vitale, N. et al. GIT proteins, A novel family of phosphatidylinositol 3,4,5-trisphosphate-stimulated GTPase-activating proteins for ARF6. J. Biol. Chem. 275, 13901–13906 (2000).
Zhang, Q., Cox, D., Tseng, C.C., Donaldson, J.G. & Greenberg, S. A requirement for ARF6 in Fcγ receptor-mediated phagocytosis in macrophages. J. Biol. Chem. 273, 19977–19981 (1998).
Li, S. et al. Chemoattractant-stimulation Rac activation in wild-type and Rac2-deficient murine neutrophils: preferential activation of Rac2 and Rac2 gene dosage effect on neutrophil functions. J. Immunol. 169, 5043–5051 (2002).
Filippi, M.D. et al. Localization of Rac2 via the C terminus and aspartic acid 150 specifies superoxide generation, actin polarity and chemotaxis in neutrophils. Nat. Immunol. 5, 744–751 (2004).
Roberts, A.W. et al. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity 10, 183–196 (1999).
Glogauer, M. et al. Rac1 deletion in mouse neutrophils has selective effects on neutrophil functions. J. Immunol. 170, 5652–5657 (2003).
Sun, C.X. et al. Rac1 is the small GTPase responsible for regulating the neutrophil chemotaxis compass. Blood 104, 3758–3765 (2004).
Roth, M.G. in GTPase 176–197 (Oxford Univ Press, New York, 2000).
Feng, Q., Baird, D. & Cerione, R.A. Novel regulatory mechanisms for the Dbl family guanine nucleotide exchange factor Cool-2/α-Pix. EMBO J. 23, 3492–3504 (2004).
Comer, F.I. & Parent, C.A. PI 3-kinases and PTEN: how opposites chemoattract. Cell 109, 541–544 (2002).
Eichinger, L. et al. The genome of the social amoeba Dictyostelium discoideum. Nature 435, 43–57 (2005).
Yagi, T. et al. A novel ES cell line, TT2, with high germline-differentiating potency. Anal. Biochem. 214, 70–76 (1993).
Suda, Y. et al. Driven by the same Ig enhancer and SV40 T promoter ras induced lung adenomatous tumors, myc induced pre-B cell lymphomas and SV40 large T gene a variety of tumors in transgenic mice. EMBO J. 6, 4055–4065 (1987).
Kitamura, H. et al. IL-6-STAT3 controls intracellular MHC class II αβ dimmer level through cathepsin S activity in dendritic cells. Immunity 23, 491–502 (2005).
Bellocchio, S. et al. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J. Immunol. 172, 3059–3069 (2004).
Grocott, R.G. A stain for fungi in tissue sections and smears using Gomori's methenamine-silver nitrate technic. Am. J. Clin. Pathol. 25, 975–979 (1955).
Romano, M. et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 6, 315–325 (1997).
Mócsai, A., Ligeti, E., Lowell, C.A. & Berton, G. Adhesion-dependent degranulation of neutrophils requires the Src family kinases Fgr and Hck. J. Immunol. 162, 1120–1126 (1999).
Lowell, C.A., Fumagalli, L. & Berton, G. Deficiency of Src family kinases p59/61hck and p58c-fgr results in defective adhesion-dependent neutrophil functions. J. Cell Biol. 133, 895–910 (1996).
Mayer, B.J., Hirai, H. & Sakai, R. Evidence that SH2 domains promote processive phosphorylation by protein-tyrosine kinases. Curr. Biol. 5, 296–305 (1995).
Hirai, K., Moriguchi, K. & Wang, G.Y. Human neutrophils produce free radicals from the cell-zymosan interface during phagocytosis and from the whole plasma membrane when stimulated with calcium ionophore A23187. Exp. Cell Res. 194, 19–27 (1991).
Gakidis, M.A. et al. Vav GEFs are required for β2 integrin-dependent functions of neutrophils. J. Cell Biol. 166, 273–282 (2004).
Luton, F. et al. EFA6, exchange factor for ARF6, regulates the actin cytoskeleton and associated tight junction in response to E-cadherin engagement. Mol. Biol. Cell 15, 1134–1145 (2004).
Benard, V. & Bokoch, G.M. Assay of Cdc42, Rac, and Rho GTPase activation by affinity methods. Methods Enzymol. 345, 349–359 (2002).
Acknowledgements
We thank M. Hiraishi, Y. Shibata and M. Iwahara for help; H.A. Popiel (Osaka University, Suita, Japan) for critical reading of the manuscript; and R.Y. Tsien (University of California, La Jolla, California) for monomeric red fluorescent protein. Supported by Grants-In-Aid from the Ministry of Education, Science, Sports and Culture of Japan (16370090 to H.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Targeting of the Git2 gene by homologous recombination (PDF 287 kb)
Supplementary Fig. 2
Requirement of GAP activity for GIT2 function. (PDF 474 kb)
Supplementary Fig. 3
Subcellular localization of Rac1 and Rac2. (PDF 2124 kb)
Supplementary Table 1
Blood parameters of Git2+/− and Git2−/− mice. (PDF 17 kb)
Supplementary Table 2
DNA sequence of PCR primers. (PDF 25 kb)
Rights and permissions
About this article
Cite this article
Mazaki, Y., Hashimoto, S., Tsujimura, T. et al. Neutrophil direction sensing and superoxide production linked by the GTPase-activating protein GIT2. Nat Immunol 7, 724–731 (2006). https://doi.org/10.1038/ni1349
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1349
This article is cited by
-
An Intriguing Structural Modification in Neutrophil Migration Across Blood Vessels to Inflammatory Sites: Progress in the Core Mechanisms
Cell Biochemistry and Biophysics (2024)
-
LRRK2 is involved in the chemotaxis of neutrophils and differentiated HL-60 cells, and the inhibition of LRRK2 kinase activity increases fMLP-induced chemotactic activity
Cell Communication and Signaling (2023)
-
ARF1 recruits RAC1 to leading edge in neutrophil chemotaxis
Cell Communication and Signaling (2017)
-
In Vitro Oxidation of Collagen Promotes the Formation of Advanced Oxidation Protein Products and the Activation of Human Neutrophils
Inflammation (2016)
-
Proteome changes in the small intestinal mucosa of broilers (Gallus gallus) induced by high concentrations of atmospheric ammonia
Proteome Science (2015)