Abstract
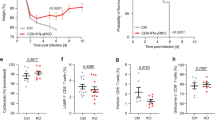
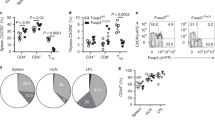
Infection with Leishmania major induces a protective immune response and long-term resistance to reinfection, which is thought to depend upon persistent parasites. Here we demonstrate that although effector CD4+ T cells are lost in the absence of parasites, central memory CD4+ T cells are maintained. Upon secondary infection, these central memory T cells become tissue-homing effector T cells and mediate protection. Thus, immunity to L. major is mediated by at least two distinct populations of CD4+ T cells: short-lived pathogen-dependent effector cells and long-lived pathogen-independent central memory cells. These data suggest that central memory T cells should be the targets for nonlive vaccines against infectious diseases requiring cell-mediated immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Reiner, S.L. & Locksley, R.M. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13, 151–177 (1995).
Sacks, D.L. & Noben-Trauth, N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2, 845–858 (2002).
Afonso, L.C. et al. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science 263, 235–237 (1994).
Gurunathan, S., Prussin, C., Sacks, D.L. & Seder, R.A. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat. Med. 4, 1409–1415 (1998).
Gurunathan, S., Klinman, D.M. & Seder, R.A. DNA vaccines: immunology, application, and optimization. Annu. Rev. Immunol. 18, 927–974 (2000).
Gicheru, M.M. et al. Vervet monkeys vaccinated with killed Leishmania major parasites and interleukin-12 develop a type 1 immune response but are not protected against challenge infection. Infect. Immun. 69, 245–251 (2001).
Rhee, E.G. et al. Vaccination with heat-killed Leishmania antigen or recombinant leishmanial protein and CpG oligodeoxynucleotides induces long-term memory CD4+ and CD8+ T cell responses and protection against Leishmania major infection. J. Exp. Med. 195, 1565–1573 (2002).
Desjeux, P. Leishmaniasis. Public health aspects and control. Clin. Dermatol. 14, 417–423 (1996).
Uzonna, J.E., Wei, G., Yurkowski, D. & Bretscher, P. Immune elimination of Leishmania major in mice: implications for immune memory, vaccination, and reactivation disease. J. Immunol. 167, 6967–6974 (2001).
Belkaid, Y., Piccirillo, C.A., Mendez, S., Shevach, E.M. & Sacks, D.L. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420, 502–507 (2002).
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712. (1999).
Masopust, D., Vezys, V., Marzo, A.L. & Lefrancois, L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291, 2413–2417 (2001).
Reinhardt, R.L., Khoruts, A., Merica, R., Zell, T. & Jenkins, M.K. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410, 101–105 (2001).
Roman, E. et al. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J. Exp. Med. 196, 957–968 (2002).
Seder, R.A. & Ahmed, R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat. Immunol. 4, 835–842 (2003).
Kaech, S.M., Hemby, S., Kersh, E. & Ahmed, R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111, 837–851 (2002).
Wherry, E.J. et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4, 225–234 (2003).
Bradley, L.M., Watson, S.R. & Swain, S.L. Entry of naive CD4 T cells into peripheral lymph nodes requires L-selectin. J. Exp. Med. 180, 2401–2406 (1994).
Jung, T.M., Gallatin, W.M., Weissman, I.L. & Dailey, M.O. Down-regulation of homing receptors after T cell activation. J. Immunol. 141, 4110–4117 (1988).
Picker, L.J. et al. Differential expression of homing-associated adhesion molecules by T cell subsets in man. J. Immunol. 145, 3247–3255 (1990).
Zaph, C. & Scott, P. Th1 cell-mediated resistance to cutaneous infection with Leishmania major is independent of P- and E-selectins. J. Immunol. 171, 4726–4732 (2003).
Titus, R.G., Gueiros-Filho, F.J., de Freitas, L.A. & Beverley, S.M. Development of a safe live Leishmania vaccine line by gene replacement. Proc. Natl. Acad. Sci. USA 92, 10267–10271 (1995).
Brodskyn, C., Beverley, S.M. & Titus, R.G. Virulent or avirulent (dhfr-ts−) Leishmania major elicit predominantly a type-1 cytokine response by human cells in vitro. Clin. Exp. Immunol. 119, 299–304 (2000).
Mocci, S. & Coffman, R.L. Induction of a Th2 population from a polarized Leishmania-specific Th1 population by in vitro culture with IL-4. J. Immunol. 154, 3779–3787 (1995).
Mocci, S. & Coffman, R.L. The mechanism of in vitro T helper cell type 1 to T helper cell type 2 switching in highly polarized Leishmania major-specific T cell populations. J. Immunol. 158, 1559–1564 (1997).
Iezzi, G., Scheidegger, D. & Lanzavecchia, A. Migration and function of antigen-primed nonpolarized T lymphocytes in vivo. J. Exp. Med. 193, 987–993. (2001).
Wang, X. & Mosmann, T. In vivo priming of CD4 T cells that produce interleukin (IL)-2 but not IL-4 or interferon (IFN)-gamma, and can subsequently differentiate into IL-4- or IFN-gamma-secreting cells. J. Exp. Med. 194, 1069–1080 (2001).
Lanzavecchia, A. & Sallusto, F. Progressive differentiation and selection of the fittest in the immune response. Nat. Rev. Immunol. 2, 982–987 (2002).
Doyle, A.M. et al. Induction of cytotoxic T lymphocyte antigen 4 (CTLA-4) restricts clonal expansion of helper T cells. J. Exp. Med. 194, 893–902 (2001).
Gray, D. A role for antigen in the maintenance of immunological memory. Nat. Rev. Immunol. 2, 60–65 (2002).
Zinkernagel, R.M. On natural and artificial vaccinations. Annu. Rev. Immunol. 21, 515–546 (2003).
Swain, S.L., Hu, H. & Huston, G. Class II-independent generation of CD4 memory T cells from effectors. Science 286, 1381–1383 (1999).
Murali-Krishna, K. et al. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science 286, 1377–1381 (1999).
London, C.A., Perez, V.L. & Abbas, A.K. Functional characteristics and survival requirements of memory CD4+ T lymphocytes in vivo. J. Immunol. 162, 766–773 (1999).
Bunce, C. & Bell, E.B. CD45RC isoforms define two types of CD4 memory T cells, one of which depends on persisting antigen. J. Exp. Med. 185, 767–776 (1997).
Garcia, S., DiSanto, J. & Stockinger, B. Following the development of a CD4 T cell response in vivo: from activation to memory formation. Immunity 11, 163–171 (1999).
Harbertson, J., Biederman, E., Bennett, K.E., Kondrack, R.M. & Bradley, L.M. Withdrawal of stimulation may initiate the transition of effector to memory CD4 cells. J. Immunol. 168, 1095–1102 (2002).
Flynn, J.L. Immunology of tuberculosis and implications in vaccine development. Tuberculosis (Edinb.) 84, 93–101 (2004).
Desrosiers, R.C. Prospects for an AIDS vaccine. Nat. Med. 10, 221–223 (2004).
Scott, P., Pearce, E., Natovitz, P. & Sher, A. Vaccination against cutaneous leishmaniasis in a murine model. I. Induction of protective immunity with a soluble extract of promastigotes. J. Immunol. 139, 221–227 (1987).
Lyons, A.B. & Parish, C.R. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods 171, 131–137 (1994).
Acknowledgements
We thank K. Joyce for excellent technical assistance, members of the Department of Pathobiology for constructive discussions and D. Artis, C.G. Feng, C.A. Hunter, D. Jankovic, E.J. Pearce, S.L. Reiner, H. Shen and A. Sher for critical reading of the manuscript. This work was supported by US National Institutes of Health grant AI35914 (to P. S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Homing to LNs is not required for effector T cell function during chronic infection with L. major (PDF 59 kb)
Supplementary Fig. 2
CD4+ T cells mediate infection-induced immunity (PDF 44 kb)
Supplementary Fig. 3
Characterization of CD4+ T cells from naive and immune B6 mice prior to adoptive transfer (PDF 44 kb)
Supplementary Fig. 4
Leishmania-specific IFN-γ and IL-2 responses by CD62Lhigh and CD62Llow CD4+ T cells (PDF 42 kb)
Rights and permissions
About this article
Cite this article
Zaph, C., Uzonna, J., Beverley, S. et al. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat Med 10, 1104–1110 (2004). https://doi.org/10.1038/nm1108
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1108
This article is cited by
-
Trimeric protein vaccine based on Beta variant elicits robust immune response against BA.4/5-included SARS-CoV-2 Omicron variants
Molecular Biomedicine (2023)
-
Research progress on tumor whole-cell vaccines prepared with nanoparticles for tumor immunotherapy
Journal of Nanoparticle Research (2023)
-
Centrin-deficient Leishmania mexicana confers protection against New World cutaneous leishmaniasis
npj Vaccines (2022)
-
Evaluation of calpain T-cell epitopes as vaccine candidates against experimental Leishmania major infection: a pilot study
Parasitology Research (2022)
-
A second generation leishmanization vaccine with a markerless attenuated Leishmania major strain using CRISPR gene editing
Nature Communications (2020)