Abstract
Although over 50 years have passed since its first laboratory description, intentional induction of immune tolerance to foreign antigens has remained an elusive clinical goal. We previously reported that the requirement for ABO compatibility in heart transplantation is not applicable to infants. Here, we show that ABO-incompatible heart transplantation during infancy results in development of B-cell tolerance to donor blood group A and B antigens. This mimics animal models of neonatal tolerance and indicates that the human infant is susceptible to intentional tolerance induction. Tolerance in this setting occurs by elimination of donor-reactive B lymphocytes and may be dependent upon persistence of some degree of antigen expression. These findings suggest that intentional exposure to nonself A and B antigens may prolong the window of opportunity for ABO-incompatible transplantation, and have profound implications for clinical research on tolerance induction to T-independent antigens relevant to xenotransplantation.
Similar content being viewed by others
Main
Historically, ABO compatibility between donor and recipient has been required for successful organ transplantation. Transplantation of ABO-incompatible donor organs into recipients with preformed antibodies to donor A or B antigens usually results in 'hyperacute' or acute antibody-mediated rejection, initiated by antibody binding to graft antigens resulting in complement activation and thrombosis of graft vasculature. Transplantation of ABO-incompatible kidney and liver grafts has been accomplished with some success, but aggressive immunosuppressive strategies are required in mature individuals, often including splenectomy1,2,3,4,5,6. ABO-incompatible heart transplantation has been performed rarely, usually unintentionally, because of the high risk of lethal antibody-mediated rejection5.
In 1945, Owen defined the inherent susceptibility to immune tolerance induction during immaturity as a consequence of antigen exposure during gestation7. He reported that dizygotic twin calves exposed to allogeneic blood from shared placental circulation did not reject skin grafts from each other. Burnet then linked immune tolerance to developmental events8. Medawar and colleagues showed that tolerance could be induced intentionally ('acquired' tolerance), whereby introduction of antigens to fetal or neonatal mice resulted in permanent and specific abrogation of the development of immune responsiveness to those antigens9. Evidence emerged showing several mechanisms of neonatally induced tolerance, including functional inactivation (anergy) or deletion of immature alloreactive clones, suppression of alloreactivity by immunoregulatory cells and, in B cells, receptor editing10,11,12,13,14,15,16. Although studied extensively at both T-cell and B-cell levels in mouse models, acquired neonatal tolerance has not been previously shown in humans.
Neonatal tolerance was not generally viewed as a clinically relevant phenomenon because human immune maturation at birth is considerably more advanced than in mice, thus presumably beyond susceptibility to tolerance induction17,18. Nonetheless, the human infant manifests several aspects of immunologic immaturity, notably deficiency of humoral responsiveness to stimulation by carbohydrate ('T-independent type 2') antigens19,20,21. This includes development of isohemagglutinins or 'natural' antibodies to nonself A and B blood group antigens, which remain low during the first months of life22,23. Reasoning that humoral rejection of ABO-incompatible heart grafts would not occur in the absence of preformed donor-specific antibody, we undertook a trial of ABO-incompatible heart transplantation in infants, using standard immunosuppression without splenectomy24. There were no cases of hyperacute or acute humoral rejection, nor clinical problems attributable to blood group incompatibility. We have now studied the B-cell immunobiology of ABO-incompatible infant heart transplant recipients and, for the first time, found evidence of neonatally acquired donor-specific B-cell tolerance in humans.
Results
Ontogeny of blood group–specific antibodies
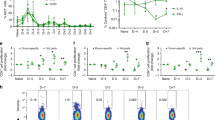
We analyzed sequential serum samples from 13 recipients of ABO-incompatible grafts and 3 recipients of ABO-compatible grafts to detect circulating A-specific and B-specific antibodies before transplantation.
Whereas serum A-specific antibodies developed normally in group O recipients of heart grafts from group B donors (B→O recipients; Table 1 and Fig. 1a) despite nonspecific immunosuppressants, we noted a persistent deficiency in circulating B-specific antibodies (Fig. 1b). Similarly, we observed a deficiency in development of A-specific antibodies in A→O recipients (patients 5–9), whereas B-specific antibodies developed normally (Fig. 1c,d). Serum A-specific and B-specific antibodies remained negligible in B→A (patients 11 and 13) and A→B (patient 12) recipients (Fig. 1e,f), whereas O→O recipients (patients 14–16) developed high titers of both A-specific and B-specific antibodies (Fig. 1g,h), similar to nonimmunosuppressed individuals. This selective deficiency in isohemagglutinin ontogeny suggests that donor-specific B-cell tolerance has occurred after ABO-incompatible transplantation, induced by exposure to donor blood group antigens during the ill-defined period of immunologic immaturity in the human infant.
Isohemagglutinin titer values indicate the inverse of the highest dilution of serum giving a positive result. Transplantation was performed at (a,b) A-specific (a) and B-specific (b) antibody development in B→O and A2B→O recipients (n = 4). c,d) A-specific (c) and B-specific (d) antibody development in A→O recipients (n = 5). (e,f) A-specific (e) and B-specific (f) antibody development in A→B, B→A and A1B→O recipients (n = 4). (g,h) A-specific (g) and B-specific (h) antibody development in O→O recipients (n = 3).
Analysis of successful ABO-incompatible adult human kidney transplants showed the phenomenon of graft accommodation, whereby grafts survived without damage despite intragraft deposition of donor-specific antibodies and complement components such as C4d3,4. Thus, low levels of circulating antibodies may reflect deposition within the graft rather than impaired production. To confirm that deficient serum donor-specific antibodies in ABO-incompatible graft recipients was not a result of intragraft deposition, we assessed endomyocardial biopsies for immunoglobulin and complement deposition in addition to routine analysis for acute rejection. In sequential biopsies obtained during follow-up ranging from 1.5 to 5 years after transplant, immunohistochemistry staining showed no deposition of immunoglobulin (IgG or IgM) or complement (C3 or C4d, or both); (Table 1 and Fig. 2a,b; one representative example is shown of a negative biopsy; comparison is made to a case of antibody-mediated rejection resulting from HLA sensitization). We did not observe any other histologic evidence of antibody-mediated graft damage attributable to ABO incompatibility. Thus, lack of circulating and intragraft antibodies confirmed impairment of antibody production against donor A and B antigens. (Note: As previously reported, the incidence of acute cellular rejection in the original cohort was slightly less in ABO-incompatible graft recipients than in ABO-compatible graft recipients24; chronic vascular rejection manifested as accelerated graft vasculopathy developed in one ABO-incompatible recipient (Table 1).
(a) Fluorescence staining for complement component C4d in a specimen from an ABO-incompatible graft. (b) Similar staining for C4d in a graft specimen from a known case of antibody-mediated rejection resulting from HLA sensitization (positive control). (c) A-antigen expression in a graft biopsy from a group A donor 2 years after transplantation into a group O recipient. Arrows indicate positive A-antigen staining. (d) Staining for B antigen in same patient as in c. (e) Staining for A antigen in a graft biopsy from a group B donor 4 years after transplantation into a group O recipient. (f) B-antigen expression in same patient as in e. Arrows indicate positive B-antigen staining.
Patients 4 and 10 (both AB→O) demonstrated divergent patterns of A-specific antibody development. Although both patients did not produce clinically significant levels of serum B-specific antibodies (Fig. 1b,f), only patient 10 remained deficient in A-specific antibody (Fig. 1a,e). Notably, patient 10 received a group A1B graft, while patient 4's donor was group A2B. The uncommon A2 subtype is characterized by low-density cell-surface A antigen expression compared to the prevalent A1 subtype25, and group A2B cells have even fewer A antigen sites than A2 cells26. In this setting, exposure to A2B graft antigens would seem to induce tolerance only to the B antigens, whereas A1B antigen exposure was tolerogenic for both A and B antigens.
Absence of agglutination-inhibiting factors
The apparent deficiency of donor-specific isohemagglutinins in sera from ABO-incompatible graft recipients (Fig. 1) could result from interference with donor-specific antibodies by serum factors, such as idiotype-specific antibodies. To assess this possibility, we devised a modified hemagglutination assay in which serum from a group O volunteer was serially diluted using patients' sera rather than saline before incubation with group A or B erythrocytes. Titers at which agglutination of group A and B erythrocytes occurred by group O serum diluted with patients' sera were equal to titers obtained with saline dilution of the same group O serum, that is, A-specific >1:256 and B-specific >1:256. Sera from all 13 ABO-incompatible recipients showed no inhibition of erythrocyte agglutination by serially diluted serum from a single group O volunteer. Thus, even at dilutions of 1:256 (group O serum:patient serum) patients' sera were incapable of preventing erythrocyte agglutination by group O serum. This supports our conclusion that donor-specific isohemagglutination deficiency in recipients of ABO-incompatible transplants results from absence of donor-specific antibody.
Donor antigen persistence
In animal models, persistence of donor antigen has been shown to be important for induction of donor-specific tolerance27,28. Using immunoperoxidase staining, we assessed endomyocardial biopsy specimens for A and B antigen expression. We analyzed at least two sequential biopsies from seven recipients of ABO-incompatible grafts and two recipients of ABO-compatible grafts; we showed expression of A or B antigen, or both, of the heart donor in all cases that were not group O (Table 1 and Fig. 2). A antigen expression is observed in a graft biopsy from a group A donor 2 years after transplantation into a group O recipient (Fig. 2c,d); likewise, persistent B antigen expression is apparent in a biopsy from a group B donor 4 years after transplantation into a group O recipient (Fig. 2e,f).
Patient 16, a group O individual, required urgent retransplantation (unrelated to ABO status) 3 d after primary ABO-incompatible transplantation (group B donor), receiving an ABO-compatible second graft (group O donor). This patient developed A-specific and B-specific antibodies in a normal pattern (Fig. 1g,h), suggesting that only transient exposure to donor antigens was insufficient to induce donor-specific antibody deficiency.
Assessment of B-cell function
To ascertain cellular mechanisms of donor-specific antibody deficiency, we performed B-cell functional and phenotypic analyses using whole blood samples from a subset of 12 ABO-incompatible and 3 ABO-compatible graft recipients. Anergic lymphocytes have been defined as antigen–specific, nonresponsive cells that can recover responsiveness after in vitro culture with appropriate stimulation29,30. The inability of mouse neonatal B lymphocytes to respond to antigens can be overcome with cytokine stimulation31,32, and antibody production by human neonatal lymphocytes in response to CD3-specific stimulation can be elicited with interleukin (IL)-2 and IL-4 (ref. 33).
To determine whether tolerance in infant recipients of ABO-incompatible grafts resulted from functionally inactive donor-specific B cells, we cultured patient-derived peripheral blood mononuclear cells (PBMC) in vitro with nonspecific cytokine or specific donor antigen stimulation, and measured specific antibody levels in culture supernatants by ELISA. Results were consistent amongst all patients tested (Table 1 and Fig. 3). A-specific IgM production by PBMC from two A→O recipients cultured without stimulation was low, comparable to PBMC from a group A control, whereas cultured PBMC from an O→O recipient exhibited modest A-specific antibody production (Fig. 3a). When cultured with IL-2 and IL-4, A-specific IgM production was markedly increased by PBMC from the O→O recipient, but remained minimal in cultures from the A→O recipients and the group A control (Fig. 3b). We obtained similar results when we cultured cells with group A erythrocytes (Fig. 3c). To evaluate the functional capacity of patients' PBMC under in vitro culture conditions, we measured antibody production to the 'third-party' carbohydrate epitope Gal-α1-3β1-4GlcNAc-R (α-gal), a major xenoantigen against which humans produce 'natural' antibodies34. Production of α-gal-specific IgM was not substantially different amongst cytokine-stimulated culture groups (Fig. 3d). Stimulated PBMC from B→O recipients showed a similar pattern of deficient B-specific IgM antibody (Table 1). IgG isotype antibodies against donor A and B antigens were absent for all patients tested (data not shown). These data suggest that donor-specific antibody deficiency in ABO-incompatible recipients does not result from functional inactivation of antigen–specific B cells.
(a) A-specific IgM isotype antibodies produced by PBMC cultured without stimulation. (b) A-specific IgM isotype antibodies produced by PBMC stimulated with IL-2 and IL-4 cytokines. (c) A-specific IgM isotype antibodies produced by PBMC stimulated with group A erythrocytes. (d) Antibodies against 'third-party' carbohydrate epitope α-gal produced by IL-2- and IL-4-stimulated PBMC.
Visualization of antibody-producing cells
To detect B cells capable of producing donor-specific antibodies, we cultured patients' PBMC with IL-2 and IL-4 stimulation. We visualized A-specific and B-specific IgM-producing cells with ELISPOT assays (Table 1 and Fig. 4). We clearly observed spot formation showing B-specific but not A-specific antibody-producing cells in PBMC from an A→O recipients (Table 1 and Fig. 4a,b) whereas we visualized A-specific but not B-specific antibody-producing cells in PBMC from B→O and A2B→O recipients (Table 1 and Fig. 4c,d). Both A-specific and B-specific antibody-producing cells were observed in PBMC from O→O recipients (Table 1 and Fig. 4e,f), although neither was visualized in PBMC from A→B, B→A and A1B→O recipients (Table 1). ELISPOT assays for IgG isotype antibody-producing cells were negative for all patients tested (data not shown).
Detection of antigen-specific B lymphocytes
These studies suggest that B-cell elimination, not anergy, is the mechanism of abrogation of B-cell responsiveness to donor A and B antigens in ABO-incompatible graft recipients. To directly ascertain the presence of donor-specific B cells, we sought B lymphocytes bearing A antigen–specific B-cell receptors (BCR) by immunostaining patients' PBMC with biotin-conjugated synthetic group A antigen and FITC-conjugated human CD19-specific monoclonal antibody (Fig. 5). FACS analysis of PBMC from an O→O recipient showed 1.8% of the total B-cell population with BCR specific for A antigen (Fig. 5a), whereas in PBMC from an A→O recipient, this B-cell population was absent (Fig. 5b), similar to the FACS profile of PBMC from a group A negative control (Fig. 5c).
Each graph contains 104 gated CD19+ cells. Percentages indicate the proportion of the CD19+ population bearing A antigen–specific BCR. Panels show analyses of PBMC from (a) an O→O recipient (positive control patient) (b) an A→O recipient and (c) a group A negative control. FITC, fluorescein isothiocyanate; PE, phyloerythrin.
Discussion
These studies of immunologic development, performed up to 8 years after ABO-incompatible infant heart transplantation, show a persistent and selective deficiency of circulating antibodies to donor A and B antigens without antibody-mediated damage. Potential explanations for antibody deficiency include: intragraft immunoglobulin deposition ('accommodation'), suppressed antibody production resulting from nonspecific systemic immunosuppression and deficient antibody production resulting from specific B-cell tolerance. Absence of immunoglobulin and complement deposition in biopsy specimens suggests that accommodation has not occurred; normal development of A-specific and B-specific antibodies in O→O infant transplant recipients indicates that immunosuppressants do not suppress isohemagglutinin development. Thus, the preponderance of evidence suggests that these children have acquired donor-specific B-cell tolerance.
Several B-cell tolerance mechanisms have been identified, including anergy (functional inactivation)29,30, deletion35,36,37 and receptor editing38,39,40. In vitro analysis of PBMC from ABO-incompatible graft recipients showed that these cells were simultaneously incapable of producing antibodies against donor-type A and B antigens, yet capable of producing antibodies against an irrelevant third-party polysaccharide antigen, and that B lymphocytes bearing BCR specific for donor-type A and B antigens were absent. These data suggest that anergy does not have a role in this setting and that antigen-specific B cells have been eliminated by deletion or receptor editing.
The site at which the tolerizing interaction occurs between immature recipient B cells and donor A and B antigens is difficult to delineate in humans, thus definitive conclusions cannot be made from these experiments. Tolerance may be induced within the graft; alternatively, or in combination, tolerizing interactions may occur in nongraft sites such as spleen or other lymphoid organs, or in bone marrow or peripheral blood, through migration of graft-derived cells or dispersal of donor antigens through perioperative administration of donor-type noncellular blood products (platelets and plasma). Several findings suggest that transient exposure to donor antigens, such as administration of noncellular blood products, is insufficient to induce tolerance. First, patient 16 (blood group O) received a group B graft for a 3-d period only and was administered group B blood products intraoperatively. Yet after receiving a group O second graft, patient 16 developed B-specific antibody production to 'normal' titers for age (Fig. 1h). Thus, transient exposure to B antigen of the first donor, whether in the graft itself or by group B blood products, was insufficient to induce tolerance. Second, patient 4 (A2B→O) received group A1B platelets and plasma products containing both A and B antigens. Yet, after transplantation with a group A2B graft, patient 4 was not rendered tolerant to A antigen contained in group A1B blood products, developing A-specific antibodies with normal kinetics (Fig. 1a).
The spleen is thought to be important for A-specific and B-specific antibody production, inasmuch as splenectomy has been reported to diminish the production of these antibodies in ABO-incompatible adult kidney transplant recipients1. But it has also been shown that splenectomy in children does not prevent development of polysaccharide-specific antibodies41, showing that production of these antibodies is not limited to the spleen. In our one case of a congenitally asplenic person (patient 6, A→O), B-specific antibody developed normally (Fig. 1d), consistent with the theory that isohemagglutinin production is not limited to the spleen, whereas A-specific antibody production did not develop at all (Fig. 1c), suggesting that interactions leading to tolerance also are not confined to the spleen.
These combined findings suggest, in accordance with animal tolerance models27,28, that persistent exposure to donor antigens is required for tolerance induction, possibly in the graft itself, and that tolerance is dependent on a certain degree of antigen expression. Consistent with these results, experiments with 3-83 (H-2k,b-specific) immunoglobulin transgenic mice showed that specific self-reactive B cells are eliminated by deletion or receptor editing upon repeated exposure to antigen-bearing cells42.
The developmental limits of susceptibility to tolerance induction to donor A and B antigens have not yet been delineated. Although patient 9 (A→O) already had established A-specific antibody production prior to transplant at age 14 months (titer 1:128; Fig. 1c), A-specific antibody was depleted by plasma exchange during the operative procedure and did not reaccumulate to clinically significant levels by 5 years after transplant, showing a persistent antibody deficiency similar to younger A→O recipients. Furthermore, A-specific antibody-producing cells could not be detected by ELISPOT assay (Table 1). Thus, acquisition of 'immune competence,' shown by antibody production before transplant, did not preclude continued susceptibility to apparent tolerance induction. This raises the possibility that the window during which ABO-incompatible transplantation could be performed might be prolonged by repeated intentional exposure to nonself A and B antigens before transplant. This single case notwithstanding, caution must be advised for consideration of ABO-incompatible transplantation in children in whom isohemagglutinin production has already begun to develop.
Both systemic immunosuppression and (neonatal) thymectomy have been shown to interfere with tolerance induction in experimental settings43,44,45,46,47. That tolerance developed in ABO-incompatible infant recipients despite these factors may be related to the fact that polysaccharide antigens such as blood group substances are considered to be 'T-cell independent'48, and most systemic immunosuppressive drugs are directed predominantly at T-cell function, which is largely mature in neonates49.
Whether tolerance to donor A and B antigens in infants is also associated with tolerance to donor HLA has not been addressed in these studies. Although tolerance may develop to donor HLA, one could also speculate that the developmental differences in maturation of human immune responses to carbohydrates versus proteins19,21 may result in susceptibility to tolerance induction to carbohydrate antigens (A and B) concomitant with 'sensitization' to protein antigens (HLA).
Neonatally induced transplantation tolerance has not been shown previously in humans. The setting of ABO-incompatible infant heart transplantation is unique in that it allows an early immune intervention and a clear strategy of assessing immunity versus tolerance to defined donor antigen(s). We have delineated the mechanism of successful ABO-incompatible infant heart transplantation as “actively acquired tolerance of foreign cells”9, and characterized here as donor antigen–specific B-cell elimination. For the first time, we have shown that human infants are sufficiently immature for intentional induction of immunologic tolerance, at least in the B-cell compartment to T-independent A and B antigens. Through understanding the mechanisms underlying tolerance in this setting, it may be possible to expand the potential applications of this strategy to older patient populations, which could result in decreased mortality for patients awaiting transplantation and increased usage of donor organs. Additionally, our findings have significance for xenogeneic transplantation in which similar issues of polysaccharide antigen and antibody production remain major obstacles to success50.
Methods
Patients and biologic samples.
We studied 16 cardiac allograft recipients between February 1996 and October 2002 (Table 1); 13 infants (median age at transplant 10.4 weeks) received grafts from ABO-incompatible donors and 3 infants (median age 20.4 weeks) received ABO-compatible grafts. Of the 16 transplant recipients, 15 recipients are clinically well at current ages ranging from 2.2 to 8.4 years; one recipient died (age 2.5 years) due to accelerated graft vasculopathy (undetectable donor-specific antibody at time of death). We reported heart transplant and immunosuppression details previously24. Near-total thymectomy was performed as a routine part of infant cardiac surgery; splenectomy was not performed. We obtained peripheral serum samples for isohemagglutinin detection at defined time points after transplant. Endomyocardial biopsies for clinical rejection surveillance were obtained at defined time points; we performed immunohistochemistry staining to detect antibody-mediated rejection and donor antigen persistence. For a subset of 15 transplant recipients (Table 1), additional whole blood samples were obtained for B-cell functional analysis. We performed experiments in compliance with institutional guidelines and approved by the Research Ethics Board of The Hospital for Sick Children. Informed consent was obtained from transplant recipients' parents.
Blood group determination and isohemagglutinin assay.
We determined blood types using standard blood bank procedures. Serum A-specific and B-specific antibody titers were measured using agglutination tests with erythrocytes of known blood type ('reverse' blood typing). We diluted patient serum with saline in ratios ranging from 1:1 to 1:256 and mixed the serum with group A or B erythrocytes. A modified version of the isohemagglutination assay was used to test for serum factors that might inhibit erythrocyte agglutination by A-specific or B-specific antibodies (Supplementary Methods online).
Immunohistochemical staining.
We stained endomyocardial biopsy sections for deposition of immunoglobulin and complement components and for persistent expression of donor A or B antigens (Supplementary Methods online) and a cardiac immunopathologist examined the sections.
Cell isolation and cultures.
We layered patient peripheral blood onto HISTOPAQUE-1077 solution (Sigma). Following centrifugation, we removed serum and stored it at −30 °C. PBMC at the serum-HISTOPAQUE interface were washed and either used immediately for cell culture or flow cytometry, or resuspended in fetal bovine serum (FBS) containing 10% dimethyl sulfoxide (Sigma) for long-term storage in liquid nitrogen. We cultured 1 × 105 cells per well in RPMI 1640 medium supplemented with 10% FBS, 5 μg/ml bovine insulin (Sigma), 5 μg/ml human transferrin (Sigma), 5 ng/ml sodium selenite, 50 μM 2-mercaptoethanol, 100 U/ml penicillin and 100 μg/ml streptomycin. For erythrocyte-stimulated cultures, 2 × 105 cells per well of washed human erythrocytes were added on the first day of culture. We did not change medium before supernatant collection. In cytokine-stimulated cultures, 200 U/ml human recombinant interleukin(IL)-2 (PeproTech Canada) and 50 U/ml human recombinant IL-4 (PeproTech Canada) were added on the first day of culture.
Enzyme-linked immunosorbent assay (ELISA).
We coated polystyrene plates (Corning) with 5 μg/ml synthetic group A or B trisaccharide conjugated to bovine serum albumin (V-Labs). Nonspecific binding sites were blocked with PBS-Tween containing 2% FBS. We cultured patients' cryopreserved PBMC with or without stimulation. After 10 d, culture supernatants were incubated in wells for 1 h; bound antibodies were detected using horseradish peroxidase–conjugated goat antibodies to human IgM or IgG (Southern Biotechnology, 1:4,000 dilution) followed by color development with 3,3′,5,5′-tetramethylbenzidine (Sigma). We stopped the reaction with sulfuric acid after 30 min and measured absorbance at 450 nm. Preparation of a standard curve allowed antibody concentration to be determined from optical density. Negative controls were PBMC from blood group A (A-specific negative control) and blood group B (B-specific negative control) volunteers; assay results for negative control samples were 0–4 ng/ml; assay results >5 ng/ml were scored as positive.
Enzyme-linked immunospot (ELISPOT) assay.
We coated nitrocellulose membranes of 96-well MultiScreen-HA plates (Millipore) with 5 μg/ml synthetic group A or B trisaccharide conjugated to bovine serum albumin. Cryopreserved PBMC were cultured for 10 d with IL-2 and IL-4. After washing, we cultured cells for 24 h in MultiScreen-HA plates and stained them with biotin-conjugated goat antibodies to human IgM or IgG (Southern Biotechnology, 1:4,000 dilution). Incubation with streptavidin-horseradish peroxidase was followed by color development with AEC solution. Negative controls, in which we did not visualize any spots, were PBMC from blood group A (A-specific negative control) and blood group B (B-specific negative control) volunteers.
Flow cytometry.
We immunostained PBMC for direct detection of B lymphocytes bearing A antigen–specific BCR. PBMC (5 × 105 cells) were incubated with 1.25 μg/ml synthetic group A tetrasaccharide-PAA-biotin conjugate (GlycoTech) and FITC-conjugated human CD19-specific monoclonal antibody (PharMingen, 10 μl/106 cells), followed by strep-tavidin-phycoerythrin. We used group A and O PBMC as negative and positive controls, respectively, with at least two samples for each experimental group. We gated CD19+ cells using FACScan flow cytometer (BD Biosciences) and analyzed data with WinMDI Version 2.8. The detection limit of group A antigen-specific BCR-bearing cells in the negative control population was approximately 0.2% of total CD19+ B cells.
Note: Supplementary information is available on the Nature Medicine website.
References
Alexandre, G.P. et al. Present experiences in a series of 26 ABO-incompatible living donor renal allografts. Transplant. Proc. 19, 4538–4542 (1987).
Sutherland, D.E. et al. Long-term effect of splenectomy versus no splenectomy in renal transplant patients. Reanalysis of a randomized prospective study. Transplantation 38, 619–624 (1984).
Bannett, A.D., McAlack, R.F., Morris, M., Chopek, M.W. & Platt, J.L. ABO incompatible renal transplantation: a qualitative analysis of native endothelial tissue ABO antigens after transplantation. Transplant. Proc. 21, 783–785 (1989).
Chopek, M.W., Simmons, R.L. & Platt, J.L. ABO-incompatible kidney transplantation: initial immunopathologic evaluation. Transplant. Proc. 19, 4553–4557 (1987).
Cooper, D.K. A clinical survey of cardiac transplantation between ABO blood group-incompatible recipients and donors. Transplant Proc. 22, 1457 (1990).
Gugenheim, J., Samuel, D., Reynes, M. & Bismuth, H. Liver transplantation across ABO blood group barriers. Lancet 336, 519–523 (1990).
Owen, R.D. Immunogenetic consequences of vascular anastomoses between bovine twins. Science 102, 400–405 (1945).
Burnet, F.M. & Fenner, F. The Production of Antibodies. (Macmillan and Company, Melbourne, 1949).
Billingham, R.E., Brent, L. & Medawar, P.B. Activity acquired tolerance of foreign cells. Nature 172, 603–606 (1953).
Dorsch, S. & Roser, B. Suppressor cells in transplantation tolerance. I. Analysis of the suppressor status of neonatally and adoptively tolerized rats. Transplantation 33, 518–524 (1982).
Kappler, J.W., Roehm, N. & Marrack, P. T cell tolerance by clonal elimination in the thymus. Cell 49, 273–280 (1987).
Klinman, N.R. The “clonal selection hypothesis” and current concepts of B cell tolerance. Immunity 5, 189–195 (1996).
McCarthy, S.A. & Bach, F.H. The cellular mechanism of maintenance of neonatally induced tolerance to H-2 class I antigens. J. Immunol. 131, 1676–1682 (1983).
Ramsdell, F. & Fowlkes, B.J. Clonal deletion versus clonal anergy: the role of the thymus in inducing self tolerance. Science 248, 1342–1348 (1990).
Ramsdell, F., Lantz, T. & Fowlkes, B.J. A nondeletional mechanism of thymic self tolerance. Science 246, 1038–1041 (1989).
Streilein, J.W. Neonatal tolerance of H-2 alloantigens. Procuring graft acceptance the “old-fashioned” way. Transplantation 52, 1–10 (1991).
West, L.J. Defining critical windows in the development of the human immune system. Hum. Exp. Toxicol. 21, 499–505 (2002).
West, L.J. Developmental aspects of immunomodulation: exploiting the immature immune system for organ transplantation. Transpl. Immunol. 9, 149–153 (2002).
Cadoz, M. Potential and limitations of polysaccharide vaccines in infancy. Vaccine 16, 1391–1395 (1998).
Gennery, A.R. et al. Effect of immunosuppression after cardiac transplantation in early childhood on antibody response to polysaccharide antigen. Lancet 351, 1778–1781 (1998).
Wilson, C.B. Immunologic basis for increased susceptibility of the neonate to infection. J. Pediatr. 108, 1–12 (1986).
Fong, S.W., Qaqundah, B.Y. & Taylor, W.F. Developmental patterns of ABO isoagglutinins in normal children correlated with the effects of age, sex., and maternal isoagglutinins. Transfusion 14, 551–559 (1974).
Springer, G.F. & Horton, R.E. Blood group isoantibody stimulation in man by feeding blood group-active bacteria. J. Clin. Invest. 48, 1280–1291 (1969).
West, L.J. et al. ABO-incompatible heart transplantation in infants. N. Engl. J. Med. 344, 793–800 (2001).
Smalley, C.E. & Tucker, E.M. Blood group A antigen site distribution and immunoglobulin binding in relation to red cell age. Br. J. Haematol. 54, 209–219 (1983).
Mollison, P.L., Contreras, M. & Engelfriet, C.P. ABO, Lewis, Ii and P groups. in Blood Transfusion in Clinical Medicine. 9th edn 155 (Blackwell Scientific Publishing, Oxford, 1993).
Hamano, K., Rawsthorne, M.A., Bushell, A.R., Morris, P.J. & Wood, K.J. Evidence that the continued presence of the organ graft and not peripheral donor microchimerism is essential for maintenance of tolerance to alloantigen in vivo in anti-CD4 treated recipients. Transplantation 62, 856–860 (1996).
Morecki, S., Leshem, B., Eid, A. & Slavin, S. Alloantigen persistence in induction and maintenance of transplantation tolerance. J. Exp. Med. 165, 1468–1480 (1987).
Erikson, J., Radic, M.Z., Camper, SA., Hardy, R.R., Carmack, C. & Weigert, M. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature 349, 331–334 (1991).
Goodnow, C.C. et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature 334, 676–682 (1988).
Chelvarajan, R.L., Gilbert, N.L. & Bondada, S. Neonatal murine B lymphocytes respond to polysaccharide antigens in the presence of IL-1 and IL-6. J. Immunol. 161, 3315–3324 (1998).
Luo, W., Fine, J., Garg, M., Kaplan, A.M. & Bondada, S. Interleukin-10 enhances immune responses to pneumococcal polysaccharides and sheep erythrocytes in young and aged mice. Cell Immunol. 195, 1–9 (1999).
Splawski, J.B. & Lipsky, P.E. Cytokine regulation of immunoglobulin secretion by neonatal lymphocytes. J. Clin. Invest. 88, 967–977 (1991).
Galili, U. Interaction of the natural anti-Gal antibody with alpha-galactosyl epitopes: a major obstacle for xenotransplantation in humans. Immunol. Today 14, 480–482 (1993).
Chen, C. et al. The site and stage of anti-DNA B-cell deletion. Nature 373, 252–255 (1995).
Hartley, S.B., Crosbie, J., Brink, R., Kantor, A.B., Basten, A. & Goodnow, C.C. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature 353, 765–769 (1991).
Nemazee, D.A. & Burki, K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature 337, 562–566 (1989).
Gay, D., Saunders, T., Camper, S. & Weigert, M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J. Exp. Med. 177, 999–1008 (1993).
Radic, M.Z., Erikson, J., Litwin, S. & Weigert, M. B lymphocytes may escape tolerance by revising their antigen receptors. J. Exp. Med. 177, 1165–1173 (1993).
Tiegs, S.L., Russell, D.M. & Nemazee, D. Receptor editing in self-reactive bone marrow B cells. J. Exp. Med. 177, 1009–1020 (1993).
Hammarstrom, L. & Smith, CI. Development of anti-polysaccharide antibodies in asplenic children. Clin. Exp. Immunol. 66, 457–462 (1986).
Lang, J. & Nemazee, D. B cell clonal elimination induced by membrane-bound self-antigen may require repeated antigen encounter or cell competition. Eur. J. Immunol. 30, 689–696 (2000).
Wang, C., Sun, J., Sheil, A.G., McCaughan, G.W. & Bishop, G.A. A short course of methylprednisolone immunosuppression inhibits both rejection and spontaneous acceptance of rat liver allografts. Transplantation 72, 44–51 (2001).
Perico, N., Imberti, O., Bontempelli, M. & Remuzzi, G. Immunosuppressive therapy abrogates unresponsiveness to renal allograft induced by thymic recognition of donor antigens. J. Am. Soc. Nephrol. 5, 1618–1623 (1995).
Yamada, K. et al. Role of the thymus in transplantation tolerance in miniature swine. I. Requirement of the thymus for rapid and stable induction of tolerance to class I-mismatched renal allografts. J. Exp. Med. 186, 497–506 (1997).
Noris, M. et al. Thymic microchimerism correlates with the outcome of tolerance-inducing protocols for solid organ transplantation. J. Am. Soc. Nephrol. 12, 2815–2826 (2001).
Nakafusa, Y., Goss, J.A. & Flye, M.W. Prevention by thymectomy of tolerance induced by intrathymic injection of donor splenocytes. Surgery 114, 183–189 (1993).
Mond, J.J., Lees, A. & Snapper, C.M. T cell-independent antigens type 2. Annu. Rev. Immunol. 13, 655–692 (1995).
Holsapple, M.P., West, L.J. & Landreth, K.S. Species comparison of anatomical and functional immune system development. Birth Defects Res. Part B Dev. Reprod. Toxicol. 68, 321–334 (2003).
Lin, S.S. et al. The role of anti-Galalpha1-3Gal antibodies in acute vascular rejection and accommodation of xenografts. Transplantation 70, 1667–1674 (2000).
Acknowledgements
We thank K. Wood and M. Sykes for discussion and advice, and P. Morris and R. Zhong for reading of our manuscript. This work was supported with funding from the Canadian Institutes for Health Research, the Heart and Stroke Foundation of Ontario, and the Research Training Competition at The Hospital for Sick Children.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Fan, X., Ang, A., Pollock-BarZiv, S. et al. Donor-specific B-cell tolerance after ABO-incompatible infant heart transplantation. Nat Med 10, 1227–1233 (2004). https://doi.org/10.1038/nm1126
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1126
This article is cited by
-
Measuring Alloreactive B Cell Responses in Transplant Recipients
Current Transplantation Reports (2019)
-
Monitoring alloimmune response in kidney transplantation
Journal of Nephrology (2017)
-
Some considerations on the current debate about typing resolution in solid organ transplantation
Transplantation Research (2016)
-
Neonatal Systemic AAV Induces Tolerance to CNS Gene Therapy in MPS I Dogs and Nonhuman Primates
Molecular Therapy (2015)
-
Strategies to overcome the ABO barrier in kidney transplantation
Nature Reviews Nephrology (2015)