Abstract
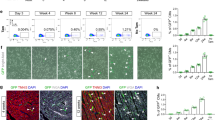
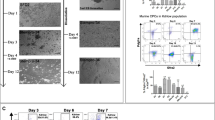
An emerging concept is that the mammalian myocardium has the potential to regenerate, but that regeneration might be too inefficient to repair the extensive myocardial injury that is typical of human disease1,2,3,4,5,6,7,8. However, the degree to which stem cells or precursor cells contribute to the renewal of adult mammalian cardiomyocytes remains controversial. Here we report evidence that stem cells or precursor cells contribute to the replacement of adult mammalian cardiomyocytes after injury but do not contribute significantly to cardiomyocyte renewal during normal aging. We generated double-transgenic mice to track the fate of adult cardiomyocytes in a 'pulse-chase' fashion: after a 4-OH-tamoxifen pulse, green fluorescent protein (GFP) expression was induced only in cardiomyocytes, with 82.7% of cardiomyocytes expressing GFP. During normal aging up to one year, the percentage of GFP+ cardiomyocytes remained unchanged, indicating that stem or precursor cells did not refresh uninjured cardiomyocytes at a significant rate during this period of time. By contrast, after myocardial infarction or pressure overload, the percentage of GFP+ cardiomyocytes decreased from 82.8% in heart tissue from sham-treated mice to 67.5% in areas bordering a myocardial infarction, 76.6% in areas away from a myocardial infarction, and 75.7% in hearts subjected to pressure overload, indicating that stem cells or precursor cells had refreshed the cardiomyocytes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jackson, K.A. et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J. Clin. Invest. 107, 1395–1402 (2001).
Beltrami, A.P. et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114, 763–776 (2003).
Oh, H. et al. Cardiac muscle plasticity in adult and embryo by heart-derived progenitor cells. Ann. NY Acad. Sci. 1015, 182–189 (2004).
Yoon, Y.S. et al. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J. Clin. Invest. 115, 326–338 (2005).
Winitsky, S.O. et al. Adult murine skeletal muscle contains cells that can differentiate into beating cardiomyocytes in vitro. PLoS Biol. 3, e87 (2005).
Laugwitz, K.L. et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 433, 647–653 (2005).
Becker, R.O., Chapin, S. & Sherry, R. Regeneration of the ventricular myocardium in amphibians. Nature 248, 145–147 (1974).
Poss, K.D., Wilson, L.G. & Keating, M.T. Heart regeneration in zebrafish. Science 298, 2188–2190 (2002).
Dor, Y., Brown, J., Martinez, O.I. & Melton, D.A. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41–46 (2004).
Sohal, D.S. et al. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ. Res. 89, 20–25 (2001).
Novak, A., Guo, C., Yang, W., Nagy, A. & Lobe, C.G. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis 28, 147–155 (2000).
Fazel, S. et al. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J. Clin. Invest. 116, 1865–1877 (2006).
Ducharme, A. et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J. Clin. Invest. 106, 55–62 (2000).
Rockman, H.A. et al. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 88, 8277–8281 (1991).
Hsieh, P.C., Davis, M.E., Gannon, J., MacGillivray, C. & Lee, R.T. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J. Clin. Invest. 116, 237–248 (2006).
Acknowledgements
P.C.H. was supported by a fellowship from the American Heart Association. V.F.M.S. was supported by a Ph.D. fellowship of the Research Foundation— Flanders (FWO) and by a Belgian American Educational Foundation research fellowship. M.E.D. was supported by an NRSA fellowship from NIH/NHLBI. This study was supported by NIH/NHLBI grant R01HL081404.
Author information
Authors and Affiliations
Contributions
Experiments were designed by P.C.H., V.F.M.S., M.E.D., J.D.M., J.R. and R.T.L. MerCreMer-ZEG mice were generated by P.C.H. and V.F.M.S., surgeries were performed by P.C.H., J.G. and V.F.M.S., immunohistochemistry and immunofluorescence staining were performed by P.C.H., V.F.M.S. and C.M., GFP+ and β-galactosidase+ cells were counted by M.E.D. and data were analyzed by P.C.H., V.F.M.S. and R.T.L. The manuscript was written by V.F.M.S., P.H.C. and R.T.L. All authors discussed the results and commented on the manuscripts.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–5, Supplementary Table 1 (PDF 558 kb)
Rights and permissions
About this article
Cite this article
Hsieh, P., Segers, V., Davis, M. et al. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med 13, 970–974 (2007). https://doi.org/10.1038/nm1618
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1618
This article is cited by
-
Return of the Tbx5; lineage-tracing reveals ventricular cardiomyocyte-like precursors in the injured adult mammalian heart
npj Regenerative Medicine (2023)
-
Piezo1 is the cardiac mechanosensor that initiates the cardiomyocyte hypertrophic response to pressure overload in adult mice
Nature Cardiovascular Research (2022)
-
Activation of Nkx2.5 transcriptional program is required for adult myocardial repair
Nature Communications (2022)
-
Neonatal injury models: integral tools to decipher the molecular basis of cardiac regeneration
Basic Research in Cardiology (2022)
-
Postnatal state transition of cardiomyocyte as a primary step in heart maturation
Protein & Cell (2022)