Abstract
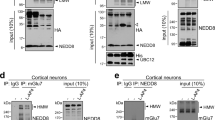
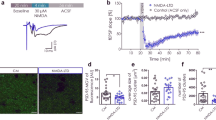
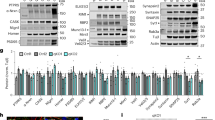
A key step in glutamatergic synapse maturation is the replacement of developmentally expressed N-methyl-D-aspartate receptors (NMDARs) with mature forms that differ in subunit composition, electrophysiological properties and propensity to elicit synaptic plasticity. However, the mechanisms underlying the removal and replacement of synaptic NMDARs are poorly understood. Here we demonstrate that NMDARs containing the developmentally regulated NR3A subunit undergo rapid endocytosis from the dendritic plasma membrane in cultured rat hippocampal neurons. This endocytic removal is regulated by PACSIN1/syndapin1, which directly and selectively binds the carboxy-terminal domain of NR3A through its NPF motifs and assembles a complex of proteins including dynamin and clathrin. Endocytosis of NR3A by PACSIN1 is activity dependent, and disruption of PACSIN1 function causes NR3A accumulation at synaptic sites. Our results reveal a new activity-dependent mechanism involved in the regulation of NMDAR expression at synapses during development, and identify a brain-specific endocytic adaptor that confers spatiotemporal and subunit specificity to NMDAR endocytosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Petralia, R.S. et al. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat. Neurosci. 2, 31–36 (1999).
Sheng, M., Cummings, J., Roldan, L.A., Jan, Y.N. & Jan, L.Y. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature 368, 144–147 (1994).
Carmignoto, G. & Vicini, S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science 258, 1007–1011 (1992).
Crair, M.C. & Malenka, R.C. A critical period for long-term potentiation at thalamocortical synapses. Nature 375, 325–328 (1995).
Hestrin, S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature 357, 686–689 (1992).
Perez-Otaño, I. & Ehlers, M.D. Learning from NMDA receptor trafficking: clues to the development and maturation of glutamatergic synapses. Neurosignals 13, 175–189 (2004).
Wenthold, R.J., Prybylowski, K., Standley, S., Sans, N. & Petralia, R.S. Trafficking of NMDA receptors. Annu. Rev. Pharmacol. Toxicol. 43, 335–358 (2003).
Das, S. et al. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature 393, 377–381 (1998).
Wong, H.K. et al. Temporal and regional expression of NMDA receptor subunit NR3A in the mammalian brain. J. Comp. Neurol. 450, 303–317 (2002).
Perez-Otaño, I. et al. Assembly with the NR1 subunit is required for surface expression of NR3A-containing NMDA receptors. J. Neurosci. 21, 1228–1237 (2001).
Sasaki, Y.F. et al. Characterization and comparison of the NR3A subunit of the NMDA receptor in recombinant systems and primary cortical neurons. J. Neurophysiol. 87, 2052–2063 (2002).
Chatterton, J.E. et al. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature 415, 793–798 (2002).
Akaaboune, M., Culican, S.M., Turney, S.G. & Lichtman, J.W. Rapid and reversible effects of activity on acetylcholine receptor density at the neuromuscular junction in vivo. Science 286, 503–507 (1999).
Philpot, B.D., Sekhar, A.K., Shouval, H.Z. & Bear, M.F. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron 29, 157–169 (2001).
Tovar, K.R. & Westbrook, G.L. Mobile NMDA receptors at hippocampal synapses. Neuron 34, 255–264 (2002).
Snyder, E.M. et al. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat. Neurosci. 4, 1079–1085 (2001).
Nong, Y. et al. Glycine binding primes NMDA receptor internalization. Nature 422, 302–307 (2003).
Li, B. et al. Differential regulation of synaptic and extra-synaptic NMDA receptors. Nat. Neurosci. 5, 833–834 (2002).
Scott, D.B., Michailidis, I., Mu, Y., Logothetis, D. & Ehlers, M.D. Endocytosis and degradative sorting of NMDA receptors by conserved membrane-proximal signals. J. Neurosci. 24, 7096–7109 (2004).
Washbourne, P., Liu, X.B., Jones, E.G. & McAllister, A.K. Cycling of NMDA receptors during trafficking in neurons before synapse formation. J. Neurosci. 24, 8253–8264 (2004).
Mu, Y., Otsuka, T., Horton, A.C., Scott, D.B. & Ehlers, M.D. Activity-dependent mRNA splicing controls ER export and synaptic delivery of NMDA receptors. Neuron 40, 581–594 (2003).
Vissel, B., Krupp, J.J., Heinemann, S.F. & Westbrook, G.L. A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nat. Neurosci. 4, 587–596 (2001).
Barria, A. & Malinow, R. Subunit-specific NMDA receptor trafficking to synapses. Neuron 35, 345–353 (2002).
Lavezzari, G., McCallum, J., Dewey, C.M. & Roche, K.W. Subunit-specific regulation of NMDA receptor endocytosis. J. Neurosci. 24, 6383–6391 (2004).
Prybylowski, K. et al. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron 47, 845–857 (2005).
Roche, K.W. et al. Molecular determinants of NMDA receptor internalization. Nat. Neurosci. 4, 794–802 (2001).
Kessels, M.M. & Qualmann, B. Syndapins integrate N-WASP in receptor-mediated endocytosis. EMBO J. 21, 6083–6094 (2002).
Plomann, M. et al. PACSIN, a brain protein that is upregulated upon differentiation into neuronal cells. Eur. J. Biochem. 256, 201–211 (1998).
Qualmann, B., Roos, J., DiGregorio, P.J. & Kelly, R.B. Syndapin I, a synaptic dynamin-binding protein that associates with the neural Wiskott-Aldrich syndrome protein. Mol. Biol. Cell 10, 501–513 (1999).
Rao, A., Kim, E., Sheng, M. & Craig, A.M. Heterogeneity in the molecular composition of excitatory postsynaptic sites during development of hippocampal neurons in culture. J. Neurosci. 18, 1217–1229 (1998).
Blanpied, T.A., Scott, D.B. & Ehlers, M.D. Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron 36, 435–449 (2002).
Racz, B., Blanpied, T.A., Ehlers, M.D. & Weinberg, R.J. Lateral organization of endocytic machinery in dendritic spines. Nat. Neurosci. 7, 917–918 (2004).
Petralia, R.S., Wang, Y.X. & Wenthold, R.J. Internalization at glutamatergic synapses during development. Eur. J. Neurosci. 18, 3207–3217 (2003).
Groc, L. et al. Differential activity-dependent regulation of the lateral mobilities of AMPA and NMDA receptors. Nat. Neurosci. 7, 695–696 (2004).
Mori, H. et al. Role of the carboxy-terminal region of the GluR epsilon2 subunit in synaptic localization of the NMDA receptor channel. Neuron 21, 571–580 (1998).
Steigerwald, F. et al. C-Terminal truncation of NR2A subunits impairs synaptic but not extrasynaptic localization of NMDA receptors. J. Neurosci. 20, 4573–4581 (2000).
Racca, C., Stephenson, F.A., Streit, P., Roberts, J.D. & Somogyi, P. NMDA receptor content of synapses in stratum radiatum of the hippocampal CA1 area. J. Neurosci. 20, 2512–2522 (2000).
Levitt-Gilmour, T.A. & Salpeter, M.M. Gradient of extrajunctional acetylcholine receptors early after denervation of mammalian muscle. J. Neurosci. 6, 1606–1612 (1986).
Cottrell, J.R., Borok, E., Horvath, T.L. & Nedivi, E. CPG2: a brain- and synapse-specific protein that regulates the endocytosis of glutamate receptors. Neuron 44, 677–690 (2004).
Simpson, F. et al. SH3-domain-containing proteins function at distinct steps in clathrin- coated vesicle formation. Nat. Cell Biol. 1, 119–124 (1999).
Da Costa, S.R. et al. Impairing actin filament or syndapin functions promotes accumulation of clathrin-coated vesicles at the apical plasma membrane of acinar epithelial cells. Mol. Biol. Cell 14, 4397–4413 (2003).
Coyle, I.P. et al. Nervous wreck, an SH3 adaptor protein that interacts with Wsp, regulates synaptic growth in Drosophila. Neuron 41, 521–534 (2004).
Qualmann, B. & Kelly, R.B. Syndapin isoforms participate in receptor-mediated endocytosis and actin organization. J. Cell Biol. 148, 1047–1062 (2000).
Fagiolini, M. et al. Separable features of visual cortical plasticity revealed by N-methyl-D-aspartate receptor 2A signaling. Proc. Natl. Acad Sci. USA 100, 2854–2859.
Roberts, E.B. & Ramoa, A.S. Enhanced NR2A subunit expression and decreased NMDA receptor decay time at the onset of ocular dominance plasticity in the ferret. J. Neurophysiol. 81, 2587–2591 (1999).
Lu, H.C., Gonzalez, E. & Crair, M.C. Barrel cortex critical period plasticity is independent of changes in NMDA receptor subunit composition. Neuron 32, 619–634 (2001).
Kato, N. & Yoshimura, H. Reduced Mg2+ block of N-methyl-D-aspartate receptor-mediated synaptic potentials in developing visual cortex. Proc. Natl. Acad. Sci. USA 90, 7114–7118 (1993).
Burgard, E.C. & Hablitz, J.J. Developmental changes in the voltage-dependence of neocortical NMDA responses. Brain Res. Dev. Brain Res. 80, 275–278 (1994).
Cho, K.O., Hunt, C.A. & Kennedy, M.B. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron 9, 929–942 (1992).
Westphal, R.S. et al. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science 285, 93–96 (1999).
Acknowledgements
We thank J.F. Wesseling for intellectual support; K. Hawk, A. Zandueta, I. López-García, C. Zhang and H. Zhang for technical help; J.R. Naranjo and M. Palczewska for support with yeast assays; and G. Augustine, T. Blanpied, J. Hernandez, B. Philpot, A. Horton and R. Mooney for critical readings of this manuscript. This work was supported by grants from the National Alliance for Research on Schizophrenia and Depression, the Marie Curie International Reintegration Grant (IRG) Program and the Unión Temporal de Empresas project (UTE) at the Centro de Investigación Médica Aplicada (CIMA; I.P.O.), Deutsche Forschungsgemeinschaft (PL233/1-1, M.P.) and the Center for Molecular Medicine (CMMC) of the University of Cologne (TP78, M.P.). Research in D.C.L.'s laboratory is supported by grants from the US National Institutes of Health (NS32742). S.J.T. is supported by grants from the US National Institutes of Health (NS46661); R.L. by grants from Junta de Comunidades de Castilla-La Mancha (SAN-04-008-00, PAI05-040); M.D.E. by grants from the US National Institutes of Health (NS39402, MH64748), the American Heart Association, the Raymond and Beverley Sackler Foundation and the Ruth K. Broad Foundation; and S.F.H. by the US National Institutes of Health (R01 NS28709) and the McKnight and Adler Foundations. M.D.E is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Internalized NR3A is transported to early endosomes. (PDF 913 kb)
Supplementary Fig. 2
Expression of NR3A and PACSIN1 in rat brain. (PDF 1096 kb)
Supplementary Fig. 3
Expression of PACSIN1 in hippocampal neurons. (PDF 2455 kb)
Supplementary Fig. 4
Proposed model for activity-dependent synaptic removal of NR3A and regulation of NMDAR maturation during development. (PDF 788 kb)
Rights and permissions
About this article
Cite this article
Pérez-Otaño, I., Luján, R., Tavalin, S. et al. Endocytosis and synaptic removal of NR3A-containing NMDA receptors by PACSIN1/syndapin1. Nat Neurosci 9, 611–621 (2006). https://doi.org/10.1038/nn1680
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1680
This article is cited by
-
Extrasynaptic NMDA receptors in acute and chronic excitotoxicity: implications for preventive treatments of ischemic stroke and late-onset Alzheimer’s disease
Molecular Neurodegeneration (2023)
-
Structural features in the glycine-binding sites of the GluN1 and GluN3A subunits regulate the surface delivery of NMDA receptors
Scientific Reports (2019)
-
Genome-wide association study of Mycobacterium avium subspecies Paratuberculosis infection in Chinese Holstein
BMC Genomics (2018)
-
Aging and amyloid β oligomers enhance TLR4 expression, LPS-induced Ca2+ responses, and neuron cell death in cultured rat hippocampal neurons
Journal of Neuroinflammation (2017)
-
Emerging roles of GluN3-containing NMDA receptors in the CNS
Nature Reviews Neuroscience (2016)