Abstract
Cognitive training is increasingly used in the treatment of schizophrenia, but it remains unknown how this training affects functional neuroanatomy. Practice on specific cognitive tasks generally leads to automaticity and decreased prefrontal cortical activity, yet broad-based cognitive training programs may avoid automaticity and increase prefrontal cortex (PFC) activity. This study used quasi-randomized, placebo-control design and pre/post neuroimaging to examine functional plasticity associated with attention and working memory-focused cognitive training in patients with schizophrenia. Twenty-one participants with schizophrenia or schizoaffective disorder split into two demographically and performance matched groups (nine scanned per group) and nine control participants were tested 6–8 weeks apart. Compared with both patient controls and healthy controls, patients receiving cognitive training increased activation significantly more in attention and working memory networks, including dorsolateral prefrontal cortex, anterior cingulate and frontopolar cortex. The extent to which activity increased in a subset of these regions predicted performance improvements. Although this study was not designed to speak to the efficacy of cognitive training as a treatment, it is the first study to show that such training can increase the ability of patients to activate the PFC regions subserving attention and working memory.
Similar content being viewed by others
INTRODUCTION
Pioneering neuroimaging studies of learning suggested that complex skill acquisition involved a process by which the prefrontal cortex had an early active role, and was then gradually supplanted by more posterior activity patterns (Fletcher et al, 1999; Hempel et al, 2004; Koch et al, 2006; Petersen et al, 1998). This suggested that attention-demanding, controlled processes were entrained into more automatic, stimulus-response mappings. The shortcoming of this automatic learning is that it is highly stimulus bound and unlikely to be applicable outside the laboratory, classroom, or clinic. Recently, this orthodox view of limited skill generalization has been challenged by studies supporting the possibility of improving executive control processes across a number of different stimuli and tasks, with corresponding increases in prefrontal cortical function (Sohlberg and Mateer, 2001). Training techniques have been shown to increase activation in prefrontal cortex to support higher cognitive loads in healthy participants (Olesen et al, 2004), stroke patients (Westerberg et al, 2007), and children with ADHD (Klingberg et al, 2005). This study evaluates whether prefrontal cortical brain activity of patients with schizophrenia could be increased in the same, stimulus-general, manner.
The wide-spread cognitive impairments in schizophrenia patients have a large impact on functional outcomes, but are often medication resistant (Green, 1996). Thus, the allure of cognitive remediation for schizophrenia has grown in recent years following reports of reduced symptom expression (Medalia et al, 1998, 2000) and improved cognitive (Spaulding et al, 1999) and work functioning (Wexler and Bell, 2005) in schizophrenia patients undergoing training on attention and working memory tasks.
Despite promising findings of the benefits of cognitive training in these treatment studies (McGurk et al, 2007), the brain mechanisms underlying these changes are only beginning to be explored. For example, cognitive training has been shown to be associated with increases in serum BDNF, which is known to have a role in neuronal development (Vinogradov et al, 2009). This suggests that behavioral changes following training may be the result of functional plasticity, as distinct from increased automated processing following repetitive practice. In non-psychiatric subjects, repetitive practice often leads to reduced activation in prefrontal regions associated with task performance despite behavioral improvements (Koch et al, 2006). Cognitive training in healthy subjects has produced a different pattern of results than training based on repetitive practice. Olesen et al (2004) have shown increased activation in middle frontal gyrus, and superior and inferior parietal cortices following cognitive training, suggesting that these techniques improve performance by increasing prefrontal cortical activity, although non-linear changes in prefrontal cortex have also been reported (Hempel et al, 2004).
Functional changes with cognitive training in schizophrenia have yet to be examined rigorously. Patients with schizophrenia show prefrontal cortical functional abnormalities when performing cognitive tasks. The predominant pattern within dorsolateral prefrontal cortex has been hypofrontal, however, some data also show patterns of increased activity with load, or cortical inefficiency, in regions inferior and anterior to dorsolateral prefrontal cortex (Minzenberg et al, 2009). Thus, it is currently unclear if behavioral improvements will be accompanied by increased activation, increased efficiency, or cortical reorganization in the various regions of prefrontal cortex that subserve executive functioning. Wexler et al (2000) presented preliminary evidence for a patient who showed normalization of function in the left inferior frontal cortex, a region of the brain involved in cognitive functions addressed by that training. Increases in activation in frontal and visual areas were also found in a subset of patients who showed the greatest behavioral improvement (Wykes et al, 2002). While these studies led the way in showing the possibility of concomitant changes in brain activity with training, they could not rule-out the possibility that they occurred only in selected patients, or that they reflected increased motivation or allegiance effects (increased desire to comply with task demands to assist in the experiment) as no active control groups were considered.
This study was designed to further investigate biological mechanisms underlying performance changes from intensive cognitive training in working memory, a cognitive domain impaired in patients with schizophrenia (Glahn et al, 2005a; Lee and Park, 2005). A group, random-effects analysis was used to evaluate the pattern of functional changes among cognitively trained patients compared with an active control condition, included to reduce confounds related to increased treatment, social contact, motivation or allegiance during testing. The final innovation of this study was to examine the generalization of function to an unpracticed domain and the specificity of the effects to the trained domains. We hypothesized that patients who receive cognitive training would show improved working memory performance and functional plasticity resulting in increased activation in prefrontal cortical regions associated with functional impairments in schizophrenia (Glahn et al, 2005a), but that patients in an active control group and untrained healthy participants would not.
MATERIALS AND METHODS
Participants
The study included 21 schizophrenia and 9 control participants. Patients were medicated outpatients recruited through community drop-in centers and a hospital psychiatric day treatment program. Patients in the study were also required to be stable, in that medication types and dosages did not change during the course of the intervention and no patients were hospitalized during the study. Control participants were recruited through newspaper and flyer advertisements. After a complete description of the study, participants provided written informed consent. The University of Minnesota Institutional Review Board approved the protocol and consent process.
Patients’ diagnosis was confirmed using the Structured Clinical Interview for DSM-IV (SCID)-patient version (First et al, 1994). Healthy control participants were screened for the presence of lifetime Axis I psychotic or mood disorders using the SCID-nonpatient version (First et al, 1996) and for the presence of a first-degree relative with schizophrenia. Potential participants were excluded for seizure disorder and history of head injury with possible neurological sequelae, current substance abuse or dependence, or a NART score indicating an IQ below 80 (Nelson and Willison, 1991). Of twenty-five patients who met criteria for inclusion in the study, four completed the pre-testing session but did not complete training or the post-test session, one because of a job opportunity and three for lack of attendance. Only 18 patients completed the neuroimaging component of the study due to MRI contra-indications or claustrophobia, though all behavioral measures were obtained outside the scanner for the other patients.
Eligible schizophrenia patients were divided into two groups; one group (N=10 total, 9 imaged) received computer-based cognitive remediation training (REM) and one group (N=11 total, 9 imaged) received cognitive behavioral social skills training (CBSST) (Granholm et al, 2005). Patients continued with ongoing treatment, which remained consistent throughout the training period. Healthy control participants (CON) received no treatment. Quasi-random group assignment was made based on demographic characteristics and initial working memory performance. Although the groups did not differ significantly on age, race, gender, or parental education, the control group achieved a significantly higher level of personal education than either patient group (F(29)=3.683, p=0.039). The global assessment of symptoms (First et al, 1995) as well as measures of symptomotology, chronicity, and anti-psychotic medication dosage showed no significant differences between the two patient groups (Table 1). There were no differences between patient groups on other medications such as lithium (1 in REM, χ2=0.955, p=0.33), anxiolytics (two in REM, χ2=2.01, p=0.156), or anti-cholinergic load (t=0.022, p=0.885) (Minzenberg et al, 2004).
Procedures
Testing protocol
Participants were tested in two identical sessions 6–8 weeks apart. Participants performed three tasks while undergoing the fMRI portion of the study—a word ‘N-back’, a picture ‘N-back’, and a lexical decision task. Despite different task demands, they were designed to be as similar as possible in presentation and response requirements to improve comparisons of brain activation across tasks. In all tasks, a series of English words selected from the English Lexicon database (Balota et al, 2002) or animal pictures appeared on the screen for 500 ms with an inter-stimulus interval of 2000 ms and participants were required to press one of two buttons identifying each stimulus as a target or a non-target with targets presented on one-third of the trials. Participants practiced all tasks before scanning to ensure comprehension.
The ‘N-back’ tasks were modifications of a common working memory task (Gevins and Cutillo, 1993). Each task included a 0-back condition used to identify brain activation related to basal cognitive task performance including encoding, deciding, and task participation and a 2-back condition requiring greater attention and executive working memory functions including updating and sequence maintenance. The word N-back task was practiced during the cognitive training sessions and was included in the testing sessions to show functional changes with practiced stimuli. The animal picture N-back was included to examine the generalization of functional changes to unpracticed stimuli. For each task, participants performed six 30-trial blocks of the 0-back and the 2-back in random order across two scan runs.
The lexical decision task was included to test specificity of functional changes, as it used cognitive functions furthest from those addressed by the training exercises but could benefit from nonspecific effects of treatment. In this task, participants were asked to identify each stimulus as a target or non-target, in which targets were defined as real, English words and non-targets were non-words. Trials were divided into blocks of ‘easy’ (average standardized accuracy of 98%) and ‘hard’ (average standardized accuracy of 88%) to parallel the cognitive loads of the working memory task. Participants performed six 30-trial blocks of the easy and hard conditions in random order across two scan runs.
Neuroimaging methods
Images were collected on 3T Siemens scanner using a standard CP head coil. Functional data were acquired using a standard EPI sequence (35 slices; TE=28; TR=2 s; flip angle=90; slice thickness=3.5 mm; base resolution=64; FOV=224). Slices were positioned along the anterior commissure–posterior commissure plane with reference to a high-resolution functional image. Image processing and analysis was carried out using FEAT (FMRI Expert Analysis Tool) Version 5.63, part of the FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). Within scan motion correction was performed using MCFLIRT (Jenkinson et al, 2002) and there were no significant group differences in movement parameters (Table 1). Pre-statistics processing included slice-timing correction using Fourier-space time-series phase-shifting; non-brain removal using BET (Smith, 2002); spatial smoothing using a Gaussian kernel of FWHM 7 mm; mean-based intensity normalization of all volumes by the same factor; and high pass temporal filtering. Independent components analysis-based blind source separation was carried out using MELODIC (Beckmann and Smith, 2004) to detect and remove artifacts and structured physiological and movement-related noise. Finally, registration to the standard MNI152 template image was carried out using FLIRT (Jenkinson et al, 2002; Jenkinson and Smith, 2001).
Training protocol
Patients in the cognitive REM condition attended up to 25 h of training in small groups over 4–6 weeks based on the approach to cognitive remediation described by Wexler and Bell (2005). Patients performed tasks designed to train attention and memory from the battery available within a computerized software package (CogPack Marker Software). This training protocol has been shown to improve memory and executive functioning in patients with schizophrenia (Sartory et al, 2005) and tasks chosen were designed to produce improved working memory and attention capacity in the treated group. In addition, patients in the REM group trained on the word N-back one to two times a week and on N-back tasks using a variety of other stimuli (such as faces) one to two times a week to support the generalization of working memory improvements. To assure that patients were adequately challenged, when patients reached 85% accuracy on a particular memory load (for example, 2-back) the memory load for that type of stimuli was then increased (for example, to 3-back). Patients in the CBSST group also attended up to 25 h of treatment but followed a manualized group therapy protocol (Granholm et al, 2005) using cognitive and behavioral therapy methods to increase patients’ skills in symptom recognition, communication, problem solving, and relapse prevention. In both conditions, the facilitators interacted with the clients throughout small group (∼4 patients) sessions: in the REM group, this mostly involved brief one-on-one discussions regarding task performance; in the CBSST condition, this interaction was in the context of the group milieu. The mean number of treatment hours did not differ across groups (Table 1).
Statistical Design
Behavioral data analysis
Accuracy (%) and reaction times (ms) were recorded for the cognitive tasks and d’ (Coombs et al, 1970) was calculated as a measure of signal detection. Change in behavioral performance was analyzed for all three tasks using a repeated measures analysis of variance (RM-ANOVA) testing the task by group by time interaction. Within this framework, group differences in change were evaluated using contrast analyses.
Functional data analysis
Time-series statistical analysis was carried out using FILM with local autocorrelation correction (Woolrich et al, 2001). To reduce the number of voxel-wise statistical comparisons, increase the power to detect group differences, and avoid bias for subsequent analyses, data from all subjects and both time points (54 sessions) were combined to identify functional regions of interest (ROIs) for each task. Z-statistic images were thresholded using a cluster correction method to correct for multiple comparisons, as determined by Z>2.3 to provide a brainwise cluster significance threshold of p=0.05 (Worsley et al, 1992). A functional ROI produced by this method was then used to constrain analyses of group by time interactions in each task (word task=19 435 voxels, picture task=17 087 voxels, lexical task=12 476 voxels). These analyses were also thresholded at Z>2.3 and cluster corrected for multiple comparisons within the smaller functionally identified ROIs only. Analyses were also performed on whole brain data. No significant group differences outside those found using the functional ROIs was noted and so reported results are for changes found within the functional ROIs.
RESULTS
Behavioral Results
An omnibus RM-ANOVA revealed no significant group differences between performance between tasks at pre-test (F(4, 48)=1.072, p=0.38) or post-test (F(4, 48)=2.073, p=0.12). Despite being matched on initial accuracy, there was a trend toward poorer signal detection (d’) for the word working memory task in the REM group at pre-test when compared with CBSST and CON (F(1, 24)=3.21, p=0.06). There was also a significant difference on lexical decision—hard performance at post-test between CON and the two patient groups (F(1, 24)=4.96, p=0.04), with the CON group showing better signal detection. Table 2 presents each groups’ d’ at each time point.
RM-ANOVA revealed that there were significant group differences in d’ change within the high cognitive loads of the three tasks (F(4, 48)=3.386, p=0.016). These group differences in performance change were stronger in both working memory tasks than the lexical decision task and were driven by greater change in the REM group than in either the CBSST or CON groups (F(1, 24)=15.522, p=0.001). In the word working memory task, the REM group showed a greater change in d’ than either other group (F(1, 24)=8.92, p=0.006). Although both REM and CON showed significant improvements at post-test (F(1, 9)=10.126, p=0.011, and F(1, 8)=12.064, p=0.008, respectively), the CBSST group did not. Furthermore, the REM compared with the CON group trended toward a greater degree of improvement (F(1, 17)=4.098, p=0.054). In the picture working memory task, REM showed greater difference in d’ than either other group (F(1, 24)=10.14, p=0.004) due to significant improvement in the REM group (F(1, 9)=21.067, p=0.001) that was not found in the other groups. There were no differences in performance change on the lexical decision task.
There was a significant difference between the REM group and the CON group (F(2, 27)=2.756, p=0.041) on change in reaction time during the word working memory task due to a nonsignificant decrease in REM RT and a nonsignificant increase in CON RT. This effect was not found in the picture working memory task. Thus, training did not lead to a shift in the speed–accuracy relationship, although signal-detection improvements in the untrained CON group may have been related to this. Correlation analyses of demographic and clinical variables showed no significant relationships with performance (p's>0.13). However, years of education was positively correlated with performance on both working memory tasks (word, r=0.42, p=0.13; animal, r=0.32, p=0.22).
Neuroimaging Results
Regions within the functional mask showing a group by time interaction were identified and changes in task activation in these regions were explored further. For the word working memory task, patients in the REM group showed a number of regions with a significant increase in activation greater than either the CBSST group or the CON group, as detailed in Table 3 and Figure 1. These included regions in the frontopolar cortex (BA10), dorsal and dorsolateral prefrontal cortex (BA8/9 and 46), and anterior cingulate gyrus (BA24/32).
Regions showing significantly greater post-test activity than pre-test activity for the REM group compared with the CBSST or the CON group in the word 2-back task and the picture 2-back task. No significant group differences between pre-test and post-test activity were found in the lexical decision task.
In the picture working memory task, patients in the REM group again showed a number of regions with a significant increase in activation greater than either the CBSST group or the CON group (Table 3 and Figure 1). These regions also included the frontopolar cortex (BA10), dorsolateral prefrontal cortex (BA6/9), and anterior cingulate gyrus (BA24/32), as well as the insula (BA13). In the lexical decision task no regions of activation change survived correction for multiple comparisons.
A logical AND operation was performed to identify regions of overlap between the activation maps generated in the word and picture N-back tasks (Table 3, Figures 2 and 3). A number of regions, including anterior cingulate, left dorsolateral prefrontal cortex, and bilateral frontopolar cortical regions, showed a significant increase in activation in the REM group but not the other two groups. Figure 2 shows the amount of functional change found in each group. Post hoc analyses within these regions of overlap reveal that greater change in activation correlated significantly with greater change in accuracy as illustrated in Figure 3: left frontopolar cortex (r=0.83, p=0.003 word; r=0.65, p=0.02 picture) and left dorsolateral prefrontal cortex (PFC) (r=0.50, p=0.04 word; r=0.42, p=0.05 picture) showed significant functional–behavioral correlations for both word and picture working memory; right frontopolar cortex activation increased significantly with picture working memory performance but not word working memory (r=0.38, p=0.08 word; r=0.60, p=0.03 picture). There was no significant relationship between brain activity and demographic or clinical variables in these regions of interest (p's>0.17). However, there were moderate-sized correlations between age of first hospitalization and left dorsolateral (r=0.35, p=0.17) and right frontopolar (r=0.32, p=0.23) activation and between time since last hospitalization and anterior cingulate (r=0.34, p=0.19).
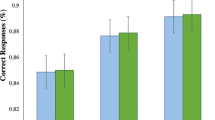
Change in functional activity in the regions of overlap in the 3-way interaction (load by time by group) between both the word and picture working memory tasks showing that in each region there is an increase in activity in the cognitive remediation group. (Effect size d for the difference in activity between the two patient groups at baseline were as follows: (a) Left dorsolateral PFC: word=−0.33; animal=0.56. (b) Anterior Cingulate: word=−0.40; animal=−0.17. (c) Left Dorsal PFC: word=0.13; animal=0.48 (d) Right Frontopolar PFC: word=−0.57; animal=0.09. (e) Left Frontopolar: word=0.01; animal=−0.55, in which a positive effect size mean REM had greater baseline difference in 2B-0B).
Regions of overlap in the three-way interaction (load by time by group) between both the word and picture working memory tasks showing activity increases in bilateral frontopolar regions, anterior cingulate cortex, and left dorsolateral/dorsal prefrontal cortex. Significant correlations between improved behavioral performance (x axis, percentage of increase) and increased functional activation (y axis, percentage of signal change) are also shown. Word 2-back is represented in red and picture 2-back in blue.
DISCUSSION
This study provides evidence of generalized behavioral improvement and functional activation increases in prefrontal cortical regions following cognitive training in patients with schizophrenia. These results suggest that patients receiving cognitive training show improved performance in the trained domain irrespective of the kind of stimuli but did not strongly influence abilities further from the training task. This suggests the improvement was not simply due to experience with verbal stimuli, improved attention or motivation. The improvements in signal detection were significantly greater than changes seen in patients receiving an active control treatment, and were significantly greater than retest effects in non-psychiatric control participants in the picture working memory task.
The inclusion of the active control condition provided further evidence that the functional changes can be interpreted as a result of the intervention. Only patients in the REM group trained on cognitive tasks, including the N-back. The CBSST patient control group did not show significant changes, suggesting that the training provided improvements beyond practice effects, regression to the mean, or other nonspecific factors. The normal control group provided additional information about normative performance on the task as well as practice effects. In some regions, the significant difference between functional change in the patient groups appeared partially due to a reduction in functional activity in the CBSST group. In most regions change in the CBSST group closely resembles change in the control group, supporting the notion that the reduction in activation may be related to the nonspecific practice effects of repeated testing. The significant group differences are largely because of the REM group differing from both patient and healthy control groups. In addition, while the normal controls did have more educational achievement (which is to be expected, as schizophrenia has a direct effect on educational attainment), REM performance shows that the intervention produced a functional and behavioral changes above even practice effects in the normal population.
The REM group's behavioral improvements were mirrored by functional increases in the prefrontal cortex. This work expanded on previous research showing fMRI changes following cognitive training (Wexler et al, 2000; Wykes et al, 2002) by introducing group-level analysis and showing stimulus-general yet training-specific effects. The anterior cingulate and dorsal/dorsolateral prefrontal cortices are involved in working memory maintenance and cognitive control processes and have reduced activity in patients with schizophrenia (Barch et al, 2002; Carter et al, 2001; MacDonald et al, 2000). A supplementary analysis of 2-back activity in the voxels within the mask at pretest found schizophrenia patients showed suggestive levels of impairment compared with controls for both the word (effect size δ=−0.50) and picture (effect size δ=−0.78) tasks, although this was not statistically significant. Increased functioning in these regions suggested that patients undergoing cognitive training show brain changes concomitant with the cognitive areas targeted by the training. Furthermore, three of these regions showed significant correlations between behavioral improvement and functional increase, similar to Wexler et al (2000), who also showed a correlation between working memory performance change and functional activity change.
The bilateral frontopolar regions shown to have increased activity at post-test are involved with working memory tasks as well as tasks requiring executive control like the Wisconsin Card Sort (Buchsbaum et al, 2005), emotional processing (Drobyshevsky et al, 2006), episodic encoding (Hofer et al, 2003), attentional modulation (Peterson et al, 1999), and cognitive response conflict (Fan et al, 2003). These regions also show abnormal functionality in patients with schizophrenia during working memory tasks (Johnson et al, 2006). Basic studies suggest that BA 10 is involved in sub-goal processing and integration of information (De Pisapia et al, 2007) as well as control processes underlying activation of an attentional set or ‘retrieval mode’ rather than trial by trial retrieval (Velanova et al, 2003). This literature suggests that the increased frontopolar activation was likely not due to trial-to-trial storage but rather executive control functions including increased hierarchical goal representations.
The results of this study suggest that patients show increasing functional activity in prefrontal working memory networks, rather than the decreased activity traditionally found as tasks become automated. Repetitive working memory training alone shows stimulus-specific practice effects (Sayala et al, 2006), yet we found significant functional changes that were not stimulus specific. In both tasks, improved behavioral performance was correlated with increased functional activity in a subset of the regions. Combined with the similarity of changes found in the practiced and generality tasks, these findings provide preliminary evidence of an increase in functional activity associated with working memory training. As patients with schizophrenia typically show hypofrontality when confronted with the same working memory task demands as control participants (Glahn et al, 2005b; Minzenberg et al, 2009), an increase in activation within this network, along with behavioral improvement, suggests that following training these patients are showing functional activity more similar to unaffected individuals.
This study was designed to detect underlying functional changes in brain activation rather than as a treatment study. Thus, although the findings provide evidence for a functional mechanism of plasticity underlying the effects of cognitive training in patients with schizophrenia, it was not designed to be sensitive to more subtle clinical or ‘real-world’ outcomes. Although the results are striking given the relatively short training, it is unclear from this study what the long-term consequences of training might be. The current pre-test/post-test design was also unable to fully characterize the dynamics of these changes over time. Also, the functional masks used may not cover the entire network used for task performance and may not include all regions susceptible to functional change. Finally, this study was designed to determine whether proximal generalization on working memory tasks might occur, which has historically been a central challenge in the learning theory (Singley and Anderson, 1989). Subsequent work should also examine more distal generalization using a variety of working memory tasks, measures that simulate real-world situations, complex cognitive tasks with working memory components, as well as larger samples to replicate the current pattern of findings.
In summary, this study provides preliminary evidence that increases the prefrontal cortical function in patients with schizophrenia who undergo cognitive training can be detected using fMRI and corresponded to performance improvements. These improvements were not stimulus-bound, but they were specific to training rather than the result of non-specific effects such as increased motivation. This compliments a larger literature on randomized clinical trials of cognitive training in schizophrenia. These imaging findings help to explain the mechanism of change underlying cognitive training protocols by suggesting that they lead to increases in activity in frontopolar cortex. This region of the brain is known to represent the attentional set brought online by cognitive control networks. This suggests that patients retain plasticity in brain circuitry responsible for the highest levels of cognitive processing, and reinforces the prospect of beneficial effects of cognitive training treatments in patients with schizophrenia.
References
Balota DA, Cortese MJ, Hutchinson KA, Neely JH, Nelson D, Simpson GB et al (2002). The English Lexicon Project: A web-based repository of descripting and behavioral measures for 40 481 English words and nonwords. http://elexicon.wustl.edu Washington University.
Barch DM, Csernansky JG, Conturo T, Snyder AZ (2002). Working and long-term memory deficits in schizophrenia: is there a common prefrontal mechanism? J Abnorm Psychol 111: 478–494.
Beckmann CF, Smith SM (2004). Probabilistic independent component analysis for functional magnetic resonance imagine. IEEE Trans on Medical Imaging 23: 137–152.
Buchsbaum BR, Greer S, Chang W, Berman KF (2005). Meta-Analysis of neuroimaging studies of the Wisconsin Card-Sorting Task and component processes. Hum Brain Mapp 25: 35–45.
Carter CS, MacDonald AW, Ross LL, Stenger AS (2001). Anterior cingulate cortex and impaired self-monitoring of performance in patients with schizophrenia: An event-related fMRI study. Am J Psychiatry 158: 1423–1428.
Coombs CH, Dawes RM, Tversky A (1970). The Theory of Signal Detectability. Mathematical Psychology. Prentice-Hall: Englewood Cliffs, NJ (pp. 165–201).
De Pisapia N, Slomski JA, Braver TS (2007). Functional specializations in lateral prefrontal cortex associated with the integration and segregation of information in working memory. Cereb Cortex 17: 993–1006.
Drobyshevsky A, Baumann SB, Schneider W (2006). A rapid fMRI task battery for mapping of visual, motor, cognitive, and emotional function. Neuroimage 31: 732–744.
Fan J, Flombaum JI, McCandliess BD, Thomas KM, Posner MI (2003). Cognitive and brain consequences of conflict. Neuroimage 18: 42–57.
First MB, Spitzer RL, Gibbon M, Williams JBW (1994). Structured Clinical Interview for DSM-IV Axis I Disorders, patient edition. SCID-I/P, Version 2.0. Biometrics Research Department, New York State Psychiatric Institute: New York.
First MB, Spitzer RL, Gibbon M, Williams JBW (1995). Structured Clinical Interview for DSM-IV Axis I Disorders, patient edition. (SCID-I/P) Version 2.0. Biometrics Research, New York State Psychiatric Institute: New York.
First MB, Spitzer RL, Gibbon M, Williams JBW (1996). Structured Clinical Interview for DSM-IV Axis I Disorders, nonpatient edition. (SCID-I/NP). Biometrics Research, New York State Psychiatric Institute: New York.
Fletcher P, Buchel C, Josephs O, Friston KJ, Dolan R (1999). Learning-related neuronal responses in prefronal cortex studied with functional neuroimaging. Cereb Cortex 9: 168–178.
Gevins A, Cutillo B (1993). Neuroelectric evidence for distributed processing in human working memory. Electroencephalogr Clin Neurophysiol 87: 128–143.
Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE et al (2005a). Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp 25: 60–69.
Glahn DC, Ragland JD, Adramoff A, Barrett J, Laird AR, Bearden CE et al (2005b). Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp 25: 60–69.
Granholm E, McQuaid JR, McClure FS, Auslander LA, Perivoliotis D, Pedrelli P et al (2005). A randomized controlled trial of cognitive behavioral social skills training for middle-aged and older outpatients with chronic schizophrenia. Am J Psychiatry 162: 520–529.
Green MF (1996). What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 153: 321–330.
Hempel A, Giesel FL, Caraballo NMG, Amann M, Meyer H, Wustenberg T et al (2004). Plasticity of cortical activation related to working memory during training. Am J Psychiatry 161: 745–747.
Hofer A, Weiss EM, Golaszewski SM, Siedentopf CM, Brinkhoff C, Kremser C et al (2003). An fMRI study of episodic encoding and recognition of words in patients with schizophrenia in remission. Am J Psychiatry 160: 911–918.
Jenkinson M, Bannister P, Brady M, Smith S (2002). Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17: 825–841.
Jenkinson M, Smith SM (2001). A global optimisation method for robust affine registration of brain images. Med Image Anal 5: 143–156.
Johnson MR, Morries NA, Astur RS, Calhoun VD, Mathalon DH, Kiehl KA et al (2006). A functional magnetic resonance imaging study of working memory abnormalities in schizophrenia. Biol Psychiatry 60: 11–21.
Klingberg T, Fernell E, Olesen P, Johnson M, Gustafsson P, Dahlstrom K et al (2005). Computerized training of working memory in children with ADHD—a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry 44: 177–186.
Koch K, Wagner G, von Consbruch K, Nenadic I, Schultz C, Ehle C et al (2006). Temporal changes in neural activation during practice of information retrieval from short-term memory: an fMRI study. Brain Res 1107: 140–150.
Lee J, Park S (2005). Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol 114: 599–611.
MacDonald III AW, Cohen JD, Stenger VA, Carter CS (2000). Dissociating the role of dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838.
Marker K . Cogpack. Marker Software: Ladenburg, Germany.
McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT (2007). A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry 164: 1791–1802.
Medalia A, Aluma M, Tryon W, Merriam A (1998). The effectiveness of attention training in schizophrenics. Schizophr Bull 24: 147–152.
Medalia A, Dorn H, Watras-Gans S (2000). Treating problem solving deficits on an acute psychiatric inpatient unit. Psychiatry Res 97: 79–88.
Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC (2009). Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry 66: 811–822.
Minzenberg MJ, Poole JH, Benton C, Vinogradov S (2004). Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. Am J Psychiatry 161: 116–124.
Nelson HE, Willison JR (1991). National Adult Reading Test (NART): Test Manual (2nd edn). Windsor: NFER-Nelson.
Olesen P, Westerberg H, Klingberg T (2004). Increased prefrontal and parietal activity after training of working memory. Nat Neurosci 7: 75–79.
Petersen S, van Mier H, Fiez JA, Raichle ME (1998). The effects of practice on the functional anatomy of task performance. Proc Natl Acad Sci USA 95: 853–860.
Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC (1999). An fMRI study of stroop word-color interference: evidence for cingulate subregions subserving multiple distributed attentional systems. Biol Psychiatry 45: 1237–1258.
Sartory G, Zorn C, Groetzinger G, Windgassen K (2005). Computerized cognitive remediation improves verbal learning and processing speed in schizophrenia. Schizophrenia Res 75: 219–223.
Sayala S, Sala JB, Courtney SM (2006). Increased neural efficiency with repeated performance of a working memory task is information-type dependent. Cereb Cortex 16: 609–617.
Singley MK, Anderson JR (1989). The Transfer of Cognitive Skill. Harvard University Press: Cambridge, MA.
Smith S (2002). Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155.
Sohlberg M, Mateer C (2001). Cognitive Rehabilitation: An Integrative Neuropsychological Appproach. The Guilford Press: New York.
Spaulding W, Reed D, Sullivan M, Richards C, Weiler M (1999). Effects of cognitive treatment in psychiatric rehabilitation. Schizophr Bull 25: 657–676.
Velanova K, Jacoby LI, Wheeler ME, McAvoy MP, Petersen SE, Buckner RL (2003). Functional-anatomic correlates of sustained and transient processing components engaged during controlled retrieval. J Neurosci 23: 8460–8470.
Vinogradov S, Fisher M, Holland C, Shelly W, Wolkowitz O, Mellon S (2009). Is serum brain-derived neurotrophic factor a biomarker for cognitive enhancement in schizophrenia? Biol Psychiatry 66: 549–553. In press.
Westerberg H, Jacobaeus H, Hirvikoski T, Clevberger P, Ostensson M, Bartfai A et al (2007). Computerized working memory training after stroke—a pilot study. Brain Injury 21: 21–29.
Wexler B, Anderson M, Fulbright R, Gore J (2000). Preliminary evidence of improved verbal working memory performance and normalization of task-related frontal lobe activation in schizophrenia following cognitive exercises. Am J Psychiatry 157: 1694–1697.
Wexler BE, Bell MD (2005). Cognitive remediation and vocational rehabiliation for schizoiphrenia. Schizophrenia Bull 31: 931–941.
Woolrich MW, Ripley BD, Brady JM, Smith SM (2001). Temporal autocorrelation in univariate linear modelling of fMRI data. NeuroImage 14: 1370–1386.
Worsley KJ, Evans AC, Marrett S, Neelin P (1992). A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 12: 900–918.
Wykes T, Brammer MJ, Mellers J, Bray P, Reeder C, Williams C et al (2002). Effects on the brain of a psychological treatment: cognitive remediation therapy. Functional magnetic resonance imaging in schizophrenia. Br J Psychiatry 181: 144–152.
Acknowledgements
We are grateful to all the participants for their conscientiousness and enthusiasm, and for the assistance of Melissa Johnson, Vina Goghari, Rachel Force, and Michelle Thompson from the TRiCAM Laboratory, and Linda Eckhardt from People's Incorporated (St Paul, MN) and Peggy Mattingly from Fairview University of Minnesota, Department of Psychiatry (Minneapolis, MN). Research supported by Clinical Research Feasibility Funding (CReFF) award from the University of Minnesota General Clinical Research Center, and NIH NCRR Grant number P41-008079 and a MIND Institute Grant to the University of Minnesota Center for Magnetic Resonance Research. KH was supported NIMH Grant number T32 MH 17069 and by a Graduate Research Partner Program fellowship through the University of Minnesota.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Previous Presentation: Preliminary data from this study have been presented at the Organization for Human Brain Mapping. Firenze, Italy (June 2006), International Congress for Schizophrenia Research, Colorado Springs, CO (March 2007) and Society for Research in Psychopathology, Iowa City, IA (October 2007).
Rights and permissions
About this article
Cite this article
Haut, K., Lim, K. & MacDonald, A. Prefrontal Cortical Changes Following Cognitive Training in Patients with Chronic Schizophrenia: Effects of Practice, Generalization, and Specificity. Neuropsychopharmacol 35, 1850–1859 (2010). https://doi.org/10.1038/npp.2010.52
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2010.52
Keywords
This article is cited by
-
Change in prefrontal activity and executive functions after action-based cognitive remediation in bipolar disorder: a randomized controlled trial
Neuropsychopharmacology (2021)
-
Cognitive Training for Military Application: a Review of the Literature and Practical Guide
Journal of Cognitive Enhancement (2019)
-
Working memory and processing speed training in schizophrenia: study protocol for a randomized controlled trial
Trials (2016)
-
A debate on working memory and cognitive control: can we learn about the treatment of substance use disorders from the neural correlates of anorexia nervosa?
BMC Psychiatry (2016)
-
Neuroplastic Changes Following Social Cognition Training in Schizophrenia: A Systematic Review
Neuropsychology Review (2016)