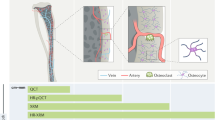
Abstract
Fluorescent microspheres are commonly used to assess bone blood supply in large animals, but the technique is not widely used in smaller mammals, as traditional methods such as reference blood sampling, ventilation and catheterization are not easily applied. This protocol describes a viable alternative for measuring bone and organ perfusion in mice using modified fluorescent microsphere techniques. Microspheres are injected directly into the left heart and a reference tissue is used to calculate relative bone and organ blood supply. On the basis of a sample of 15 mice with 5 tissues each, the entire protocol takes 140.5 h to complete from animal preparation through statistical analysis. This timing includes 72 h of mandated pauses for bone decalcification and digestion, as well as 48 h for data analysis. Exclusive of pauses or additional analyses that could increase the time required, this protocol takes 20.5 h bench time to complete.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Tothill, P., Hooper, G., Hughes, S.P.F. & McCarthy, I.D. Bone blood flow measured with microspheres: the problem of non-entrapment. Clin. Phys. Physiol. Meas. 8, 51–55 (1987).
Fluorescent Microsphere Resource Center. Manual for Using Fluorescent Microspheres to Measure Regional Organ Perfusion (University of Washington, Seattle, Washington, 1999).
Quintana, A., Raczka, E. & Bonaccorsi, A. Cardiac output distribution measured with radioactive microspheres in the mouse. Pharmacol. Res. Commun. 11, 245–252 (1979).
Glenny, R.W., Bernard, S. & Brinkley, M. Validation of fluorescent-labeled microspheres for measurement of regional organ perfusion. J. Appl. Physiol. 74, 2585–2597 (1993).
Tan, W., Riggs, K.W., Theis, R.L. & Rurak, D.W. Use of an automated fluorescent microsphere method to measure regional blood flow in the fetal lamb. Can. J. Physiol. Pharmacol. 75, 959–968 (1997).
Prinzen, F.W. & Bassingthwaighte, J.B. Blood flow distribution by microsphere deposition methods. Cardiovasc. Res. 45, 13–21 (2000).
Anetzberger, H., Thein, E., Becker, M., Zwissler, B. & Messmer, K. Microspheres accurately predict regional bone blood flow. Clin. Orthop. Relat. Res. 424, 253–265 (2004).
Anetzberger, H., Thein, E., Maier, M., Birkenmaier, C. & Messmer, K. Fluorescent microspheres are reliable for serial bone blood flow measurements. Clin. Orthop. Relat. Res. 427, 241–248 (2004).
Anetzberger, H., Thein, E., Becker, M., Walli, A.K. & Messmer, K. Validity of fluorescent microspheres method for bone blood flow measurement during intentional arterial hypotension. J. Appl. Physiol. 95, 1153–1158 (2003).
Fuchs, H. et al. The German Mouse Clinic: a platform for systemic phenotypic analysis of mouse models. Curr. Pharm. Biotechnol. 10, 236–243 (2009).
Serrat, M.A., King, D. & Lovejoy, C.O. Temperature regulates limb length in homeotherms by directly modulating cartilage growth. Proc. Natl. Acad. Sci. USA 105, 19347–19352 (2008).
Hedrich, H.J. & Bullock, G. The Laboratory Mouse (Elsevier Academic Press, Boston, Massachusetts, 2004).
Anetzberger, H., Thein, E., Löffler, G. & Messmer, K. Fluorescent microsphere method is suitable for chronic bone blood flow measurement—a long term study after meniscectomy in rabbits. J. Appl. Physiol. 96, 1928–1936 (2004).
Tarnavski, O. et al. Mouse cardiac surgery: comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol. Genomics 16, 349–360 (2004).
Mitzner, W., Lee, W., Georgakopoulos, D. & Wagner, E. Angiogenesis in the mouse lung. Am. J. Pathol. 157, 93–101 (2000).
Sarin, S.K., Sabba, C. & Groszmann, R.J. Splanchnic and systemic hemodynamics in mice using a radioactive microsphere technique. Am. J. Physiol. 258, G365–G369 (1990).
Barbee, R.W., Perry, B.D., Ré, R.N. & Murgo, J.P. Microsphere and dilution techniques for the determination of blood flows and volumes in conscious mice. Am. J. Physiol. 263, R728–R733 (1992).
Richer, C., Domergue, V., Gervais, M., Bruneval, P. & Giudicelli, J.-F. Fluospheres for cardiovascular phenotyping genetically modified mice. J. Cardiovasc. Pharmacol. 36, 396–404 (2000).
Jacobi, J. et al. Adenoviral gene transfer with soluble vascular endothelial growth factor receptors impairs angiogenesis and perfusion in a murine model of hindlimb ischemia. Circulation 110, 2424–2429 (2004).
Esaki, J. et al. Local sustained release of prostaglandin e(1) induces neovascularization in murine hindlimb ischemia. Circ. J. 73, 1330–1336 (2009).
Aardal, N.P., Svanes, K. & Egenberg, K.E. Effect of hypothermia and pentobarbital anaesthesia on the distribution of cardiac output in rabbits. Eur. Surg. Res. 5, 372 (1973).
Bennett, B.T., Brown, M.J. & Schofield, J.C. Essentials for Animal Research: A Primer for Research Personnel (National Agriculture Library, Beltsville, Maryland, 1994).
Tothill, P. & MacPherson, J.N. The distribution of blood flow to the whole skeleton in dogs, rabbits and rats measured with microspheres. Clin. Phys. Physiol. Meas. 7, 117–123 (1986).
Prisby, R.D. et al. Aging reduces skeletal blood flow, endothelium-dependent vasodilation and nitric oxide bioavailability in rats. J. Bone Miner. Res. 22, 1280–1288 (2007).
Raab, S., Thein, E., Harris, A.G. & Messmer, K. A new sample-processing unit for the fluorescent microsphere method. Am. J. Physiol. 276, H1801–H1806 (1999).
Acknowledgements
This research was supported by a grant from the National Science Foundation (BCS-0524899). W. Horne, J. Jacobi, D. Rurak, R. Glenny and the Fluorescent Microsphere Resource Center provided valuable input on microsphere injection and recovery techniques, and A. Carey (Sefar America) helped obtain essential filtration material. Special thanks to O. Lovejoy, D. King, W.E. Horton, C. Vinyard, J. Stalvey, R. Meindl, P. Reno, C. Farnum and two anonymous reviewers for critical suggestions and to D. McBurney, B. Armfield, E. Bailey, N. Smallwood and J. Hardwick for advice and technical assistance in developing this protocol.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Serrat, M. Measuring bone blood supply in mice using fluorescent microspheres. Nat Protoc 4, 1749–1758 (2009). https://doi.org/10.1038/nprot.2009.190
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.190
This article is cited by
-
Quantitative assessment of myocardial blood flow and extracellular volume fraction using 68Ga-DOTA-PET: A feasibility and validation study in large animals
Journal of Nuclear Cardiology (2020)
-
Reversal of Osteopenia in Ovariectomized Rats by Pentoxifylline: Evidence of Osteogenic and Osteo-Angiogenic Roles of the Drug
Calcified Tissue International (2019)
-
Assessment of regional pulmonary blood flow using 68Ga-DOTA PET
EJNMMI Research (2017)
-
Blood flow controls bone vascular function and osteogenesis
Nature Communications (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.