Abstract
Transplantable tumors are an accepted gold standard in cancer studies in rodents. The progress of this model in zebrafish has long been constrained by the lack of true inbred lines in zebrafish. We have generated several lines of homozygous diploid clonal zebrafish lines, which allow serial transplantations of tumor cells from one fish to another without sublethal γ-irradiation. The spectrum of transplantable tumors that were initially induced and maintained in inbred clonal zebrafish lines was limited to different types of spontaneous and diethylnitrosamine-induced hepatic tumors. However, this model can readily be extended to a broad range of extrahepatic tumors, transgenic tumors with defined mechanisms of induction and fluorescence-tagged tumor lines. These models will further facilitate in-depth analysis of invasive tumor growth, angiogenesis, metastasis and tumor-initiating cells by in vivo imaging and provide a cost-effective system for high-throughput (HTP) screening of anticancer therapeutics, including biological response modifiers. In addition, homozygous zebrafish lines are an indispensable tool for immunogenetics, mapping of quantitative trait loci and other genetic applications. The whole procedure, from generation of a gynogenetic female homozygous fish (a founder) to obtaining 3–4 consecutive passages of a syngeneic tumor, takes ∼12–18 months. This time-frame largely depends on methods of tumor induction, tumor type and tumor growth rate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Amatruda, J.F., Shepard, J.L., Stern, H.M. & Zon, L.I. Zebrafish as a cancer model system. Cancer Cell 1, 229–231 (2002).
Trevarrow, B. & Robison, B. Genetic backgrounds, standard lines and husbandry of zebrafish. Methods Cell Biol. 77, 599–615 (2004).
Streisinger, G., Walker, C., Dower, N., Knauber, D. & Singer, F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature 291, 293–296 (1981).
Streisinger, G., Singer, F., Walker, C., Knauber, D. & Dower, N. Segregation analyses and gene–centromere distances in zebrafish. Genetics 112, 311–319 (1986).
Buth, D.G., Gordon, M.S., Plaut, I., Drill, S.L. & Adam, L.G. Genetic heterogeneity in isogenic homozygous clonal zebrafish. Proc. Natl. Acad. Sci. USA 92, 12367–12369 (1995).
Nechiporuk, A., Finney, J.E., Keating, M.T. & Johnson, S.L. Assessment of polymorphism in zebrafish mapping strains. Genome Res. 9, 1231–1238 (1999).
Guryev, V. et al. Genetic variation in the zebrafish. Genome Res. 16, 491–497 (2006).
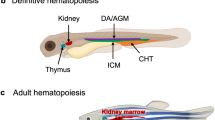
Davidson, A.J. & Zon, L.I. The 'definitive' (and 'primitive') guide to zebrafish hematopoiesis. Oncogene 23, 7233–7246 (2004).
Langenau, D.M. et al. In vivo tracking of T cell development, ablation, and engraftment in transgenic zebrafish. Proc. Natl. Acad. Sci. USA 101, 7369–7374 (2004).
Trede, N.S., Langenau, D.M., Traver, D., Look, A.T. & Zon, L.I. The use of zebrafish to understand immunity. Immunity 20, 367–379 (2004).
Mizgireuv, I.V. & Revskoy, S.Y. Transplantable tumor lines generated in clonal zebrafish. Cancer Res. 66, 3120–3125 (2006).
Khudoley, V.V. Use of aquarium fish Danio rerio and Poecilia reticulata as a test species for evaluation of nitrosamine carcinogenicity. J. Natl. Cancer Inst. Monogr. 65, 65–70 (1980).
Spitsbergen, J.M. & Kent, M.L. The state of the art of the zebrafish model for toxicology and toxicologic pathology research—advantages and current limitations. Toxicol. Pathol. 31 (Suppl): 62–87 (2003).
Lam, S.H. et al. Conservation of gene expression signatures between zebrafish and human liver tumors and tumor progression. Nat. Biotechnol. 24, 73–75 (2006).
Khannaand, C. & Hunter, K. Modeling metastasis in vivo . Carcinogenesis 26, 513–523 (2005).
Bilby, M.C. Transplantable tumours in mice—the way forward. In Relevance of tumor models for cancer drug development Contrib. Oncol. Vol. 54 (eds. Fiebig, H.H., Burger, A.M.), pp 1–13 (Basel, Karger, 1999).
Reynolds, C.P., Sun, B-C., DeClerck, Y.A. & Moats, R.A. Assessing growth and response to therapy in murine tumor models. Methods Mol. Med. 111, 335–350 (2005).
Berghmans, S. et al. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc. Natl. Acad. Sci. USA 102, 407–412 (2005).
Langenau, D.M. et al. Myc-induced T cell leukemia in transgenic zebrafish. Science 299, 887–890 (2003).
Patton, E.E. et al. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr. Biol. 15, 249–254 (2005).
Yang, H.W. et al. Targeted expression of human MYCN selectively causes pancreatic neuroendocrine tumors in transgenic zebrafish. Cancer Res. 64, 7256–7262 (2004).
Langenau, D.M. et al. Effects of RAS on the genesis of embryonal rhabdomyosarcoma. Genes Dev. 21, 1382–1395 (2007).
White, R.M. et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2, 183–189 (2008).
Stoletov, K., Montel, V., Lester, R.D., Gonias, S.L. & Klemke, R. High-resolution imaging of the dynamic tumor cell–vascular interface in transparent zebrafish. Proc. Natl. Acad. Sci. USA 104, 17406–17411 (2007).
Stoletov, K. & Klemke, R. Catch of the day: zebrafish as a human cancer model. Oncogene 27, 4509–4520 (2008).
Haldi, M., Ton, C., Seng, W.L. & McGrath, P. Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis 9, 139–151 (2005).
Topczewska, J.M. et al. Embryonic and tumorigenic pathways converge via nodal signaling: role in melanoma aggressiveness. Nat. Med. 12, 925–932 (2006).
Nicoli, S. & Presta, M. The zebrafish/tumor xenograft angiogenesis assay. Nat. Protoc. 2, 2918–2923 (2007).
Mizgireuv, I.V., Majorova, I.G., Gorodinskaya, V.M., Khudoley, V.V. & Revskoy, S.Y. Carcinogenic effect of N-nitrosodimethylamine on diploid and triploid zebrafish (Danio rerio). Toxicol. Pathol. 32, 514–518 (2004).
Gestl, E.E., Kauffman, E.J., Moore, J.L. & Cheng, K.C. New conditions for generation of gynogenetic half-tetrad embryos in the zebrafish, Danio rerio. J. Heredity 88, 76–79 (1997).
Kimmel, C.B. Genetics and early development of zebrafish. Trends Genet. 5, 283–288 (1989).
Westerfield, M. The Zebrafish Book. A guide for the laboratory use of zebrafish (Danio rerio), 5th edn. (University of Oregon Press, Eugene, OR, 2007).
Swenberg, J.A. et al. 04-Ethyldeoxythymidine, but not 06-ethyldeoxyguanosine, accumulates in hepatocyte DNA of rats exposed continuously to diethylnitrosamine. Proc. Natl. Acad. Sci. USA 81, 1692–1695 (1984).
Acknowledgements
This work was supported in part by NCI grant CA139311 (S.R.). We thank the anonymous reviewers for their very constructive comments which provided insight and expertise that greatly assisted in making this protocol more detailed and accurate.
Author information
Authors and Affiliations
Contributions
I.M. and S.R. contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Mizgirev, I., Revskoy, S. Generation of clonal zebrafish lines and transplantable hepatic tumors. Nat Protoc 5, 383–394 (2010). https://doi.org/10.1038/nprot.2010.8
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2010.8
This article is cited by
-
Immune gene variation associated with chromosome-scale differences among individual zebrafish genomes
Scientific Reports (2023)
-
A fish is not a mouse: understanding differences in background genetics is critical for reproducibility
Lab Animal (2021)
-
A 13-plex of tetra- and penta-STRs to identify zebrafish
Scientific Reports (2020)
-
Zebrafish breeding program: genetic parameters estimates for growth traits
Journal of Applied Genetics (2019)
-
Development of a novel zebrafish xenograft model in ache mutants using liver cancer cell lines
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.