Abstract
The perception of adipose tissue has changed considerably with the dramatic increase in the incidence of obesity and obesity-related comorbidities over the past 3 decades. Excess fat is no longer associated with wealth, but is instead recognized as a risk factor for many diseases. Adipose tissue is increasingly being identified as a vital, complex endocrine organ, and not simply as a fat store. Not all fat is created equal—regional, developmental, structural, and functional variations exist. Epicardial adipose tissue is a metabolically active organ producing a number of factors that modulate cardiac structure and function. The global epidemic of obesity and metabolic syndrome imposes a major disease burden, particularly of cardiovascular disease. In this Review, we describe the various types of adipose tissue—their developmental biology, differentiation, cell heterogeneity, and functional characteristics. We discuss the link between adipose tissue and inflammation, the signaling factors released by adipose tissue, as well as cardiac adiposity and its relevance to cardiovascular diseases. Finally, we review the myocardial regenerative potential of adipose-tissue-derived stem cells. We believe that a thorough understanding of adipose tissue is of great clinical value.
Key Points
-
Distinct functional types of adipose tissue exist, each with a characteristic origin and pattern of development and differentiation
-
Adipose tissue displays both structural and regional heterogeneity
-
Adipose tissue secretes numerous factors, including cytokines and growth factors, with physiological and pathological roles
-
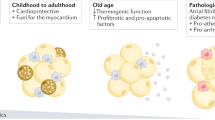
Cardiac adiposity is a marker of, and contributory factor to, various cardiac diseases
-
Adipose-tissue-derived stem cells might have an important role in myocardial regeneration and repair
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Aiello, L. C. & Wheeler, P. The expensive-tissue hypothesis—the brain and the digestive system in human and primate evolution. Curr. Anthropol. 36, 199–221 (1995).
Flier, J. S., Cook, K. S., Usher, P. & Spielgelman, B. M. Severely impaired adipsin expression in genetic and acquired obesity. Science 237, 405–408 (1987).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994).
Zuk, P. A. et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 7, 211–228 (2001).
Navarrete, A., Schaik, C. P. V. & Isler, K. Energetics and the evolution of human brain size. Nature 480, 91–93 (2011).
Ronti, T., Lupattelli, G. & Mannarino, E. The endocrine function of adipose tissue: an update. Clin. Endocrinol. (Oxf.) 64, 355–365 (2006).
Rosen, E. D. & Spiegelman, B. M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444, 847–853 (2006).
Trayhurn, P. Adipocyte biology. Obes. Rev. 8 (Suppl. 1), 41–44 (2007).
Lidell, M. E. & Enerbäck, S. Brown adipose tissue—a new role in humans? Nat. Rev. Endocrinol. 6, 319–325 (2010).
Yang, X., Enerbäck, S. & Smith, U. Reduced expression of FOXC2 and brown adipogenic genes in human subjects with insulin resistance. Obes. Res. 11, 1182–1191 (2003).
Cypess, A. M. et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 (2009).
Boström, P. et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–469 (2012).
Wu, J. et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 (2012).
Castillo-Quan, J. I. From white to brown fat through the PGC-1α-dependent myokine irisin: implications for diabetes and obesity. Dis. Model. Mech. 5, 293–295 (2012).
Poissonnet, C. M., LaVelle, M. & Burdi, A. R. Growth and development of adipose tissue. J. Pediatr. 113, 1–9 (1988).
Wajchenberg, B. L. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr. Rev. 21, 697–738 (2000).
Poissonnet, C. M., Burdi, A. R. & Garn, S. M. The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum. Dev. 10, 1–11 (1984).
Bartness, T. J., Vaughan, C. H. & Song, C. K. Sympathetic and sensory innervation of brown adipose tissue. Int. J. Obes. (Lond.) 34 (Suppl. 1), S36–S42 (2010).
Billon, N. et al. The generation of adipocytes by the neural crest. Development 134, 2283–2292 (2007).
Enerbäck, S. The origins of brown adipose tissue. N. Engl. J. Med. 360, 2021–2023 (2009).
Seale, P. et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967 (2008).
Rosen, E. D., Walkey, C. J., Puigserver, P. & Spiegelman, B. M. Transcriptional regulation of adipogenesis. Genes Dev. 14, 1293–1307 (2000).
Salma, N., Xiao, H. & Imbalzano, A. N. Temporal recruitment of CCAAT/enhancer-binding proteins to early and late adipogenic promoters in vivo. J. Mol. Endocrinol. 36, 139–151 (2006).
Darlington, G. J., Ross, S. E. & MacDougald, O. A. The role of C/EBP genes in adipocyte differentiation. J. Biol. Chem. 273, 30057–30060 (1998).
Mori, T. et al. Role of Krüppel-like factor 15 (KLF15) in transcriptional regulation of adipogenesis. J. Biol. Chem. 280, 12867–12875 (2005).
Gray, S. et al. The Krüppel-like factor KLF15 regulates the insulin-sensitive glucose transporter GLUT4. J. Biol. Chem. 277, 34322–34328 (2002).
Oishi, Y. et al. Krüppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab. 1, 27–39 (2005).
Wu, J., Srinivasan, S. V., Neumann, J. C. & Lingrel, J. B. The KLF2 transcription factor does not affect the formation of preadipocytes but inhibits their differentiation into adipocytes. Biochemistry 44, 11098–11105 (2005).
Lemieux, S. et al. Seven-year changes in body fat and visceral adipose tissue in women. Association with indexes of plasma glucose-insulin homeostasis. Diabetes Care 19, 983–991 (1996).
Tankó, L. B., Bagger, Y. Z., Alexandersen, P., Larsen, P. J. & Christiansen, C. Peripheral adiposity exhibits an independent dominant antiatherogenic effect in elderly women. Circulation 107, 1626–1631 (2003).
Porter, S. T. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care 32, 1068–1075 (2009).
Tchkonia, T. et al. Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. Am. J. Physiol. Endocrinol. Metab. 288, E267–E277 (2005).
Sethi, J. K. & Vidal-Puig, A. J. Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J. Lipid Res. 48, 1253–1262 (2007).
Alessi, M.-C., Poggi, M. & Juhan-Vague, I. Plasminogen activator inhibitor-1, adipose tissue and insulin resistance. Curr. Opin. Lipidol. 18, 240–245 (2007).
Fujioka, D. et al. Role of adiponectin receptors in endothelin-induced cellular hypertrophy in cultured cardiomyocytes and their expression in infarcted heart. Am. J. Physiol. Heart Circ. Physiol. 290, H2409–H2416 (2006).
Ouchi, N. et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein, adiponectin. Circulation 100, 2473–2476 (1999).
Ouchi, N. et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation 103, 1057–1063 (2001).
Chen, H., Montagnani, M., Funahashi, T., Shimomura, I. & Quon, M. J. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J. Biol. Chem. 278, 45021–45026 (2003).
Iacobellis, G., Petrone, A., Leonetti, F. & Buzzetti, R. Left ventricular mass and +276 G/G single nucleotide polymorphism of the adiponectin gene in uncomplicated obesity. Obesity 14, 368–372 (2006).
Considine, R. V. et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 334, 292–295 (1996).
Lee, Y. et al. Hyperleptinemia prevents lipotoxic cardiomyopathy in acyl CoA synthase transgenic mice. Proc. Natl Acad. Sci. USA 101, 13624–13629 (2004).
Smith, C. C. T. et al. Leptin-induced cardioprotection involves JAK/STAT signaling that may be linked to the mitochondrial permeability transition pore. Am. J. Physiol. Heart Circ. Physiol. 299, H1265–H1270 (2010).
Halestrap, A. P., Clarke, S. J. & Javadov, S. A. Mitochondrial permeability transition pore opening during myocardial reperfusion—a target for cardioprotection. Cardiovasc. Res. 61, 372–385 (2004).
Boengler, K., Hilfiker-Kleiner, D., Drexler, H., Heusch, G. & Schulz, R. The myocardial JAK/STAT pathway: from protection to failure. Pharmacol. Ther. 120, 172–185 (2008).
Bełtwski, J. Leptin and atherosclerosis. Atherosclerosis 189, 47–60 (2006).
Shirasaka, T., Takasaki, M. & Kannan, H. Cardiovascular effects of leptin and orexins. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R639–R651 (2003).
Heinonen, M. V. et al. Apelin, orexin-A and leptin plasma levels in morbid obesity and effect of gastric banding. Regul. Pept. 130, 7–13 (2005).
Scimia, M. C. et al. APJ acts as a dual receptor in cardiac hypertrophy. Nature 488, 394–398 (2012).
Szokodi, I. et al. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ. Res. 91, 434–440 (2002).
Tatemoto, K. et al. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul. Pept. 99, 87–92 (2001).
Leeper, N. J. et al. Apelin prevents aortic aneurysm formation by inhibiting macrophage inflammation. Am. J. Physiol. Heart Circ. Physiol. 296, H1329–H1335 (2009).
van der Veer, E. et al. Pre-B-cell colony-enhancing factor regulates NAD+-dependent protein deacetylase activity and promotes vascular smooth muscle cell maturation. Circ. Res. 97, 25–34 (2005).
Adya, R., Tan, B. K., Chen, J. & Randeva, H. S. Nuclear factor-κB induction by visfatin in human vascular endothelial cells: its role in MMP-2/9 production and activation. Diabetes Care 31, 758–760 (2008).
Smith, J., Al-Amri, M., Sniderman, A. & Cianflone, K. Visfatin concentration in Asian Indians is correlated with high density lipoprotein cholesterol and apolipoprotein A1. Clin. Endocrinol. (Oxf.) 65, 667–672 (2006).
Dahl, T. B. et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation 115, 972–980 (2007).
Lim, S. Y. et al. The novel adipocytokine visfatin exerts direct cardioprotective effects. J. Cell. Mol. Med. 12, 1395–1403 (2008).
Steppan, C. M. et al. The hormone resistin links obesity to diabetes. Nature 409, 307–312 (2001).
Azuma, K. et al. Correlation between serum resistin level and adiposity in obese individuals. Obes. Res. 11, 997–1001 (2003).
Youn, B.-S. et al. Plasma resistin concentrations measured by enzyme-linked immunosorbent assay using a newly developed monoclonal antibody are elevated in individuals with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 89, 150–156 (2004).
Lee, J. H. et al. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J. Clin. Endocrinol. Metab. 88, 4848–4856 (2003).
Frankel, D. S. et al. Resistin, adiponectin, and risk of heart failure the Framingham offspring study. J. Am. Coll. Cardiol. 53, 754–762 (2009).
Weikert, C. et al. Plasma resistin levels and risk of myocardial infarction and ischemic stroke. J. Clin. Endocrinol. Metab. 93, 2647–2653 (2008).
Kawanami, D. et al. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine–endothelial cell interactions. Biochem. Biophys. Res. Commun. 314, 415–419 (2004).
Verma, S. et al. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation 108, 736–740 (2003).
Wittamer, V. et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 198, 977–985 (2003).
Lehrke, M. et al. Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary atherosclerosis. Eur. J. Endocrinol. 161, 339–344 (2009).
Berg, A. H., Lin, Y., Lisanti, M. P. & Scherer, P. E. Adipocyte differentiation induces dynamic changes in NF-κB expression and activity. Am. J. Physiol. Endocrinol. Metab. 287, E1178–E1188 (2004).
Rajala, M. W. & Scherer, P. E. Minireview: The adipocyte—at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology 144, 3765–3773 (2003).
Fain, J. N., Madan, A. K., Hiler, M. L., Cheema, P. & Bahouth, S. W. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 145, 2273–2282 (2004).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Berg, A. H. & Scherer, P. E. Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 96, 939–949 (2005).
Dhurandhar, N. V. A framework for identification of infections that contribute to human obesity. Lancet Infect. Dis. 11, 963–969 (2011).
Atkinson, R. L. et al. Human adenovirus-36 is associated with increased body weight and paradoxical reduction of serum lipids. Int. J. Obes. (Lond.) 29, 281–286 (2005).
Iacobellis, G. & Willens, H. J. Echocardiographic epicardial fat: a review of research and clinical applications. J. Am. Soc. Echocardiogr. 22, 1311–1319 (2009).
Iacobellis, G. & Bianco, A. C. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol. Metab. 22, 450–457 (2011).
Iacobellis, G., Corradi, D. & Sharma, A. M. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat. Clin. Pract. Cardiovasc. Med. 2, 536–543 (2005).
Sacks, H. S. & Fain, J. N. Human epicardial adipose tissue: a review. Am. Heart J. 153, 907–917 (2007).
Ho, E. & Shimada, Y. Formation of the epicardium studied with the scanning electron microscope. Dev. Biol. 66, 579–585 (1978).
Moore, K. L. & Persaud, T. V. N. The developing human: clinically oriented embryology. 7th edn 189 (Saunders, Philadelphia, PA, 2003).
Iacobellis, G. & Barbaro, G. The double role of epicardial adipose tissue as pro- and anti-inflammatory organ. Horm. Metab. Res. 40, 442–445 (2008).
Sacks, H. S. et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J. Clin. Endocrinol. Metab. 94, 3611–3615 (2009).
Marchington, J. M. & Pond, C. M. Site-specific properties of pericardial and epicardial adipose tissue: the effects of insulin and high-fat feeding on lipogenesis and the incorporation of fatty acids in vitro. Int. J. Obes. 14, 1013–1022 (1990).
Paolisso, G. et al. Association of fasting plasma free fatty acid concentration and frequency of ventricular premature complexes in nonischemic non-insulin-dependent diabetic patients. Am. J. Cardiol. 80, 932–937 (1997).
Mazurek, T. et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 108, 2460–2466 (2003).
Hirata, Y. et al. Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. J. Am. Coll. Cardiol. 58, 248–255 (2011).
Iglesias, M. et al. Gender differences in adiponectin and leptin expression in epicardial and subcutaneous adipose tissue: findings in patients undergoing cardiac surgery [Spanish]. Rev. Esp. Cardiol. 59, 1252–1260 (2006).
Lauer, M. N. et al. AGT, PAI and resistin gene expression in human epicardial fat [abstract 100]. Presented at the 38th Annual Meeting of the European Association for the Study of Diabetes.
Taslipina, A. et al. Epicardial adipose tissue thickness and serum visfatin levels in patients with new diagnosed prediabetes and type 2 diabetes mellitus [abstract]. Endocrine Abstracts 20, P369 (2009).
Gollasch, M. Vasodilator signals from perivascular adipose tissue. Br. J. Pharmacol. 165, 633–642 (2012).
Iacobellis, G. et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J. Clin. Endocrinol. Metab. 88, 5163–5168 (2003).
Iacobellis, G. & Leonetti, F. Epicardial adipose tissue and insulin resistance in obese subjects. J. Clin. Endocrinol. Metab. 90, 6300–6302 (2005).
Iacobellis, G., Singh, N., Wharton, S. & Sharma, A. M. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity 16, 1693–1697 (2008).
Natale, F. et al. Visceral adiposity and arterial stiffness: echocardiographic epicardial fat thickness reflects, better than waist circumference, carotid arterial stiffness in a large population of hypertensives. Eur. J. Echocardiogr. 10, 549–555 (2009).
Eroglu, S. et al. Epicardial adipose tissue thickness by echocardiography is a marker for the presence and severity of coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 19, 211–217 (2009).
Ahn, S.-G. et al. Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart 94, e7 (2008).
Sarin, S. et al. Clinical significance of epicardial fat measured using cardiac multislice computed tomography. Am. J. Cardiol. 102, 767–771 (2008).
Alexopoulos, N. et al. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis 210, 150–154 (2010).
Janik, M. et al. Epicardial adipose tissue volume and coronary artery calcium to predict myocardial ischemia on positron emission tomography-computed tomography studies. J. Nucl. Cardiol. 17, 841–847 (2010).
de Vos, A. M. et al. Peri-coronary epicardial adipose tissue is related to cardiovascular risk factors and coronary artery calcification in post-menopausal women. Eur. Heart J. 29, 777–783 (2008).
Flüchter, S. et al. Volumetric assessment of epicardial adipose tissue with cardiovascular magnetic resonance imaging. Obesity 15, 870–878 (2007).
Corradi, D. et al. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc. Pathol. 13, 313–316 (2004).
Iacobellis, G., Leonetti, F., Singh, N. & Sharma, A. M. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int. J. Cardiol. 115, 272–273 (2007).
Al Chekakie, M. O. et al. Pericardial fat is independently associated with human atrial fibrillation. J. Am. Coll. Cardiol. 56, 784–788 (2010).
Abed, H. S. et al. Periatrial fat volume is predictive of atrial fibrillation severity [abstract PO5-24]. Heart Rhythm 7 (Suppl.), S327 (2010).
Batal, O. et al. Left atrial epicardial adiposity and atrial fibrillation. Circ. Arrhythm. Electrophysiol. 3, 230–236 (2010).
Mathieu, P., Després, J. P. & Pibarot, P. The 'valvulo-metabolic' risk in calcific aortic valve disease. Can. J. Cardiol. 23 (Suppl. B), 32B–39B (2007).
Briand, M. et al. Metabolic syndrome is associated with faster degeneration of bioprosthetic valves. Circulation 114 (Suppl. 1), I512–I517 (2006).
Lombardi, R. & Marian, A. J. Molecular genetics and pathogenesis of arrhythmogenic right ventricular cardiomyopathy: a disease of cardiac stem cells. Pediatr. Cardiol. 32, 360–365 (2011).
Rubin, J. P., Bennett, J. M., Doctor, J. S., Tebbets, B. M. & Marra, K. G. Collagenous microbeads as a scaffold for tissue engineering with adipose-derived stem cells. Plast. Reconstr. Surg. 120, 414–424 (2007).
Schipper, B. M., Marra, K. G., Zhang, W., Donnenberg, A. D. & Rubin, J. P. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann. Plast. Surg. 60, 538–544 (2008).
Fraser, J. K., Wulur, I., Alfonso, Z. & Hedrick, M. H. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 24, 150–154 (2006).
Zuk, P. A. et al. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 13, 4279–4295 (2002).
Rangappa, S. et al. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann. Thorac. Surg. 75, 775–779 (2003).
Fischer, L. J. et al. Endothelial differentiation of adipose-derived stem cells: effects of endothelial cell growth supplement and shear force. J. Surg. Res. 152, 157–166 (2009).
Brzoska, M., Geiger, H., Gauer, S. & Baer, P. Epithelial differentiation of human adipose tissue-derived adult stem cells. Biochem. Biophys. Res. Commun. 330, 142–150 (2005).
Corre, J. et al. Human subcutaneous adipose cells support complete differentiation but not self-renewal of hematopoietic progenitors. J. Cell. Physiol. 208, 282–288 (2006).
Seo, M. J., Suh, S. Y., Bae, Y. C. & Jung, J. S. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem. Biophys. Res. Commun. 328, 258–264 (2005).
Timper, K. et al. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem. Biophys. Res. Commun. 341, 1135–1140 (2006).
Rehman, J. et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109, 1292–1298 (2004).
Kim, W.-S., Park, B.-S. & Sung, J.-H. The wound-healing and antioxidant effects of adipose-derived stem cells. Expert Opin. Biol. Ther. 9, 879–887 (2009).
Cai, L. et al. IFATS collection: Human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells 27, 230–237 (2009).
Bai, X. et al. Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. Eur. Heart J. 31, 489–501 (2010).
Lapatina, M. et al. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS ONE 6, e17899 (2011).
Bai, X. et al. Genetically selected stem cells from human adipose tissue express cardiac markers. Biochem. Biophys. Res. Commun. 353, 665–671 (2007).
Gaustad, K. G., Boquest, A. C., Anderson, B. E., Gerdes, A. M. & Collas, P. Differentiation of human adipose tissue stem cells using extracts of rat cardiomyocytes. Biochem. Biophys. Res. Commun. 314, 420–427 (2004).
Planat-Bénard, V. et al. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ. Res. 94, 223–229 (2004).
van Dijk, A., Niessen, H. W. M., Zandieh Doulabi, B., Visser, F. C. & van Milligen, F. J. Differentiation of human adipose-derived stem cells towards cardiomyocytes is facilitated by laminin. Cell Tissue Res. 334, 457–467 (2008).
Planat-Bénard, V. et al. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ. Res. 94, 223–229 (2004).
Madonna, R., Willerson, J. T. & Geng, Y.-J. Myocardin a enhances telomerase activities in adipose tissue mesenchymal cells and embryonic stem cells undergoing cardiovascular myogenic differentiation. Stem Cells 26, 202–211 (2008).
Okura, H. et al. Cardiomyoblast-like cells differentiated from human adipose tissue-derived mesenchymal stem cells improve left ventricular dysfunction and survival in a rat myocardial infarction model. Tissue Eng. Pt C Methods 16, 417–425 (2010).
Valina, C. et al. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur. Heart J. 28, 2667–2677 (2007).
Bayes-Genis, A. et al. Human progenitor cells derived from cardiac adipose tissue ameliorate myocardial infarction in rodents. J. Mol. Cell. Cardiol. 49, 771–780 (2010).
Zhu, X.-Y. et al. Transplantation of adipose-derived stem cells overexpressing hHGF into cardiac tissue. Biochem. Biophys. Res. Commun. 379, 1084–1090 (2009).
Wang, L. et al. Adipose-derived stem cells are an effective cell candidate for treatment of heart failure: an MR imaging study of rat hearts. Am. J. Physiol. Heart Circ. Physiol. 297, H1020–H1031 (2009).
Schenke-Layland, K. et al. Adipose tissue-derived cells improve cardiac function following myocardial infarction. J. Surg. Res. 153, 217–223 (2009).
Langer, R. & Vacanti, J. P. Tissue engineering. Science 260, 920–926 (1993).
Wang, C. et al. A small diameter elastic blood vessel wall prepared under pulsatile conditions from polyglycolic acid mesh and smooth muscle cells differentiated from adipose-derived stem cells. Biomaterials 31, 621–630 (2010).
Colazzo, F. et al. Extracellular matrix production by adipose-derived stem cells: implications for heart valve tissue engineering. Biomaterials 32, 119–127 (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
Colman, E. et al. The FDA's assessment of two drugs for chronic weight management. N. Engl. J. Med. 367, 1577–1579 (2012).
Borrell, B. Weight-loss drug wins US approval: obesity treatment shows promise for patients with diabetes despite concerns that it could cause heart complications. Nat. News http://dx.doi.org/10.1038/nature.2012.10923.
Bal, N. C. et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat. Med. 18, 1575–1579 (2012).
Bordicchia, M. et al. Cardiac natriuretic peptides act via P38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Invest. 122, 1022–1036 (2012).
Espiritu, D. J. & Mazzone, T. Oxidative stress regulates adipocyte apolipoprotein E and suppresses its expression in obesity. Diabetes 57, 2992–2998 (2008).
Horowitz, J. F. Fatty acid mobilization from adipose tissue during exercise. Trends Endocrinol. Metab. 14, 386–392 (2003).
Zechner, R. et al. The role of lipoprotein lipase in adipose tissue development and metabolism. Int. J. Obes. Relat. Metab. Disord. 24 (Suppl. 4), S53–S56 (2000).
Badeau, M., Vihma, V., Mikkola, T. S., Tiitinen, A. & Tikkanen, M. J. Estradiol fatty acid esters in adipose tissue and serum of pregnant and pre- and postmenopausal women. J. Clin. Endocrinol. Metab. 92, 4327–4331 (2007).
Fain, J. N. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam. Horm. 74, 443–477 (2006).
Lönnqvist, F. et al. Leptin secretion from adipose tissue in women. Relationship to plasma levels and gene expression. J. Clin. Invest. 99, 2398–2404 (1997).
Garruti, G. et al. Expression and secretion of the atrial natriuretic peptide in human adipose tissue and preadipocytes. Obesity 15, 2181–2189 (2007).
Wilkison, W. O., Choy, L. & Spiegelman, B. M. Biosynthetic regulation of monobutyrin, an adipocyte-secreted lipid with angiogenic activity. J. Biol. Chem. 266, 16886–16891 (1991).
Gabrielsson, B. G. et al. High expression of complement components in omental adipose tissue in obese men. Obes. Res. 11, 699–708 (2003).
Napolitano, A. et al. Concentrations of adipsin in blood and rates of adipsin secretion by adipose tissue in humans with normal, elevated and diminished adipose tissue mass. Int. J. Obes. Relat. Metab. Disord. 18, 213–218 (1994).
Klöting, N. et al. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab. 6, 79–87 (2007).
Mohamed-Ali, V. et al. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am. J. Physiol. Endocrinol. Metab. 277, E971–E975 (1999).
Kamei, N. et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J. Biol. Chem. 281, 26602–26614 (2006).
Hoffstedt, J., Arvidsson, E., Sjölin, E., Wåhlén, K. & Arner, P. Adipose tissue adiponectin production and adiponectin serum concentration in human obesity and insulin resistance. J. Clin. Endocrinol. Metab. 89, 1391–1396 (2004).
Boucher, J. et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 146, 1764–1771 (2005).
Cai, R.-C. et al. Expression of omentin in adipose tissues in obese and type 2 diabetic patients [Chinese]. Zhonghua Yi Xue Za Zhi 89, 381–384 (2009).
Simon, M. F. et al. Lysophosphatidic acid inhibits adipocyte differentiation via lysophosphatidic acid 1 receptor-dependent down-regulation of peroxisome proliferator-activated receptor γ2. J. Biol. Chem. 280, 14656–14662 (2005).
Do, M.-S. et al. Metallothionein gene expression in human adipose tissue from lean and obese subjects. Horm. Metab. Res. 34, 348–351 (2002).
Alessi, M. C. et al. Production of plasminogen activator inhibitor 1 by human adipose tissue: possible link between visceral fat accumulation and vascular disease. Diabetes 46, 860–867 (1997).
Bełtwski, J. Adiponectin and resistin—new hormones of white adipose tissue. Med. Sci. Monit. 9, RA55–RA61 (2003).
Ding, J. et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Clin. Nutr. 90, 499–504 (2009).
Greif, M. et al. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 29, 781–786 (2009).
Tamarappoo, B. et al. Increased pericardial fat volume measured from noncontrast CT predicts myocardial ischemia by SPECT. JACC Cardiovasc. Imaging 3, 1104–1112 (2010).
Miao, C. et al. The association of pericardial fat with coronary artery plaque index at MR imaging: the Multi-Ethnic Study of Atherosclerosis (MESA). Radiology 261, 109–115 (2011).
Patil, H. et al. Non-alcoholic fatty liver disease but not epicardial fat is associated with coronary artery calcification [abstract]. J. Am. Coll. Cardiol. 59, E1345 (2012).
Mazurek, M. et al. Peri-coronary epicardial adipose tissue affects coronary atherosclerosis in patients with acute myocardial infarction [abstract 560]. Circulation 118, S580–S581 (2008).
Doesch, C. et al. Epicardial adipose tissue in patients with heart failure. J. Cardiovasc. Magn. Reson. 12, 40 (2010).
Liang, K.-W. et al. MRI measured epicardial adipose tissue thickness at the right AV groove differentiates inflammatory status in obese men with metabolic syndrome. Obesity 20, 525–532 (2011).
Author information
Authors and Affiliations
Contributions
All the authors contributed substantially to researching data for the article, discussion of its content, writing the article, and reviewing and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Hassan, M., Latif, N. & Yacoub, M. Adipose tissue: friend or foe?. Nat Rev Cardiol 9, 689–702 (2012). https://doi.org/10.1038/nrcardio.2012.148
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2012.148
This article is cited by
-
Valvulogenesis of a living, innervated pulmonary root induced by an acellular scaffold
Communications Biology (2023)
-
The role of SPARC (secreted protein acidic and rich in cysteine) in the pathogenesis of obesity, type 2 diabetes, and non-alcoholic fatty liver disease
Journal of Physiology and Biochemistry (2023)
-
Renal peritumoral adipose tissue undergoes a browning process and stimulates the expression of epithelial-mesenchymal transition markers in human renal cells
Scientific Reports (2022)
-
Adipose-derived mesenchymal stem cells promote the malignant phenotype of cervical cancer
Scientific Reports (2020)
-
Applied Systems Biology—embracing molecular imaging for systemic medicine
European Journal of Nuclear Medicine and Molecular Imaging (2020)