Key Points
-
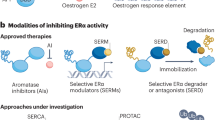
Several selective oestrogen receptor (ER) modulators and the aromatase inhibitor exemestane have been shown to reduce the incidence of ER-positive breast cancer by >50%
-
However, acceptance of these agents in the clinic is low because benefit:risk ratios are difficult to predict on an individual level and the number-needed-to-treat (NNT) is high even in 'at-risk' cohorts
-
In the future, biomarkers to assess both risk and treatment efficacy will be needed to lower the NNT in the prevention setting; similarly, communication of risk and benefit estimates must be improved
-
To accelerate prevention research, standardized biomarker validation studies are needed and definitive prevention trials should include novel end points in addition to breast cancer incidence
-
Identification of suitable biomarkers and models developed using these approaches will help reduce the size of cohorts and follow-up durations needed in prevention studies and improve patient acceptance
Abstract
Effective chemoprevention of oestrogen receptor (ER)-positive breast cancer has been shown convincingly using several selective ER modulators and the aromatase inhibitor exemestane. Although these agents are well tolerated and the numbers needed-to-treat in the prevention setting are similar to other established preventive interventions, uptake has been poor in clinical practice because of difficulties in visualizing risk, predicting individual outcomes and measuring treatment benefit. In addition, new agents targeting ER-negative breast cancer are urgently needed. The development of new agents is hampered by the lack of suitable biomarkers and targets, as well as regulatory and financial considerations. Establishing breast cancer chemoprevention in standard clinical practice will require advances in many different fields, including biomarker research, the development of more powerful tools to predict and communicate the risks and benefits of treatments and establishing innovative trial designs. Furthermore, changes in regulatory procedures could reduce the size and cost of trials needed in the prevention setting. Identification of biomarkers for risk and efficacy that are easily accessible, such as blood-based biomarkers, will be key to future chemoprevention strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Estimated cancer incidence, mortality, prevalence and disability-adjusted life years (DALYs) worldwide in 2008. International Agency for Research Against Cancer [online], (2008).
Mortality from breast cancer, age-standardised rate (World), all ages. International Agency for Research Against Cancer [online], (2011).
Bleyer, A. & Welch, H. G. Effect of three decades of screening mammography on breast-cancer incidence. N. Engl. J. Med. 367, 1998–2005 (2012).
Sporn, M. B. Approaches to prevention of epithelial cancer during the preneoplastic period. Cancer Res. 36, 2699–2702 (1976).
Cuzick, J. & Baum, M. Tamoxifen and contralateral breast cancer. Lancet 2, 282 (1985).
Fisher, B. & Redmond, C. New perspective on cancer of the contralateral breast: a marker for assessing tamoxifen as a preventive agent. J. Natl Cancer Inst. 83, 1278–1280 (1991).
Powles, T. et al. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet 352, 98–101 (1998).
Antoniou, A. et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am. J. Hum. Genet. 72, 1117–1130 (2003).
Antoniou, A. C. et al. RAD51 135G-->C modifies breast cancer risk among BRCA2 mutation carriers: results from a combined analysis of 19 studies. Am. J. Hum. Genet. 81, 1186–1200 (2007).
Clemons, M., Loijens, L. & Goss, P. Breast cancer risk following irradiation for Hodgkin's disease. Cancer Treat. Rev. 26, 291–302 (2000).
Goss, P. E. & Sierra, S. Current perspectives on radiation-induced breast cancer. J. Clin. Oncol. 16, 338–347 (1998).
Liu, P. H., Wang, J. D. & Keating, N. L. Expected years of life lost for six potentially preventable cancers in the United States. Prev. Med. 56, 309–313 (2013).
Dupont, W. D. & Page, D. L. Risk factors for breast cancer in women with proliferative breast disease. N. Engl. J. Med. 312, 146–151 (1985).
Hartmann, L. C. et al. Benign breast disease and the risk of breast cancer. N. Engl. J. Med. 353, 229–237 (2005).
Degnim, A. C. et al. Stratification of breast cancer risk in women with atypia: a Mayo cohort study. J. Clin. Oncol. 25, 2671–2677 (2007).
Warnberg, F., Yuen, J. & Holmberg, L. Risk of subsequent invasive breast cancer after breast carcinoma in situ. Lancet 355, 724–725 (2000).
Bodian, C. A., Perzin, K. H. & Lattes, R. Lobular neoplasia. Long term risk of breast cancer and relation to other factors. Cancer 78, 1024–1034 (1996).
Boyd, N. F. et al. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J. Natl Cancer Inst. 87, 670–675 (1995).
Zhang, Y. et al. Bone mass and the risk of breast cancer among postmenopausal women. N. Engl. J. Med. 336, 611–617 (1997).
Toniolo, P. G. et al. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J. Natl Cancer Inst. 87, 190–197 (1995).
Byrne, C. et al. Mammographic features and breast cancer risk: effects with time, age, and menopause status. J. Natl Cancer Inst. 87, 1622–1629 (1995).
Schairer, C. et al. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA 283, 485–491 (2000).
Magnusson, C. et al. Body size in different periods of life and breast cancer risk in post-menopausal women. Int. J. Cancer 76, 29–34 (1998).
Hsieh, C. C., Trichopoulos, D., Katsouyanni, K. & Yuasa, S. Age at menarche, age at menopause, height and obesity as risk factors for breast cancer: associations and interactions in an international case-control study. Int. J. Cancer 46, 796–800 (1990).
Cauley, J. A. et al. Elevated serum estradiol and testosterone concentrations are associated with a high risk for breast cancer. Study of Osteoporotic Fractures Research Group. Ann. Intern. Med. 130, 270–277 (1999).
Gail, M. H. et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J. Natl Cancer Inst. 81, 1879–1886 (1989).
Fisher, B. et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl Cancer Inst. 90, 1371–1388 (1998).
Vogel, V. G. et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 295, 2727–2741 (2006).
Cuzick, J. et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet 360, 817–824 (2002).
Goss, P. E. et al. Exemestane for breast-cancer prevention in postmenopausal women. N. Engl. J. Med. 364, 2381–2391 (2011).
Cuzick, J. et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. 381, 1827–1834 Lancet (2013).
Tyrer, J., Duffy, S. W. & Cuzick, J. A breast cancer prediction model incorporating familial and personal risk factors. Stat. Med. 23, 1111–1130 (2004).
Claus, E. B., Risch, N. & Thompson, W. D. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer 73, 643–651 (1994).
Chen, W. Y., Rosner, B. & Colditz, G. A. Moving forward with breast cancer prevention. Cancer 109, 2387–2391 (2007).
Surveillance Epidemiology and End Results. National Cancer Institute [online], (2013).
Breast Cancer Risk Assessment Tool. National Cancer Institute [online], (2011).
Baker, S. G. & Kramer, B. S. Evaluating a new marker for risk prediction: decision analysis to the rescue. Discov. Med. 14, 181–188 (2012).
Cauley, J. A. et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple outcomes of raloxifene evaluation. Breast Cancer Res. Treat. 65, 125–134 (2001).
LaCroix, A. Z. et al. Breast cancer incidence in the randomized PEARL trial of lasofoxifene in postmenopausal osteoporotic women. J. Natl Cancer Inst. 102, 1706–1715 (2010).
Powles, T. J. et al. Breast cancer incidence in postmenopausal women with osteoporosis or low bone mass using arzoxifene. Breast Cancer Res. Treat. 134, 299–306 (2012).
Freedman, A. N. et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J. Clin. Oncol. 29, 2327–2333 (2011).
Key, T., Appleby, P., Barnes, I., Reeves, G. & Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J. Natl Cancer Inst. 94, 606–616 (2002).
Blair, I. A. Analysis of estrogens in serum and plasma from postmenopausal women: past present, and future. Steroids 75, 297–306 (2010).
Stanway, S. J., Purohit, A. & Reed, M. J. Measurement of estrone sulfate in postmenopausal women: comparison of direct RIA and GC-MS/MS methods for monitoring response to endocrine therapy in women with breast cancer. Anticancer Res. 27, 2765–2767 (2007).
Mikkelsen, A. L., Borggaard, B. & Lebech, P. E. Results of serial measurement of estradiol in serum with six different methods during ovarian stimulation. Gynecol. Obstet. Invest. 41, 35–40 (1996).
Fiers, T. et al. Development of a highly sensitive method for the quantification of estrone and estradiol in serum by liquid chromatography tandem mass spectrometry without derivatization. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 893–894, 57–62 (2012).
Polyak, K. Heterogeneity in breast cancer. J. Clin. Invest. 121, 3786–3788 (2011).
Jung, S. et al. Fruit and vegetable intake and risk of breast cancer by hormone receptor status. J. Natl Cancer Inst. 105, 219–236 (2013).
Ursin, G. et al. Mammographic density and breast cancer in three ethnic groups. Cancer Epidemiol. Biomarkers Prev. 12, 332–338 (2003).
Boyd, N. F. et al. Mammographic densities as a criterion for entry to a clinical trial of breast cancer prevention. Br. J. Cancer 72, 476–479 (1995).
Boyd, N. F. et al. Mammographic density and the risk and detection of breast cancer. N. Engl. J. Med. 356, 227–236 (2007).
Chen, J. et al. Projecting absolute invasive breast cancer risk in white women with a model that includes mammographic density. J. Natl Cancer Inst. 98, 1215–1226 (2006).
Ursin, G., Pike, M. C., Spicer, D. V., Porrath, S. A. & Reitherman, R. W. Can mammographic densities predict effects of tamoxifen on the breast? J. Natl Cancer Inst. 88, 128–129 (1996).
Atkinson, C., Warren, R., Bingham, S. A. & Day, N. E. Mammographic patterns as a predictive biomarker of breast cancer risk: effect of tamoxifen. Cancer Epidemiol. Biomarkers Prev. 8, 863–866 (1999).
Cuzick, J., Warwick, J., Pinney, E., Warren, R. M. & Duffy, S. W. Tamoxifen and breast density in women at increased risk of breast cancer. J. Natl Cancer Inst. 96, 621–628 (2004).
Cigler, T. et al. Effects of the steroidal aromatase inhibitor exemestane on mammographic breast density and other end-organ functions [abstract]. Breast Cancer Res. Treat. 106 (Suppl.), a1026 (2007).
Cigler, T. et al. A randomized, placebo-controlled trial (NCIC CTG MAP.2) examining the effects of exemestane on mammographic breast density, bone density, markers of bone metabolism and serum lipid levels in postmenopausal women. Breast Cancer Res. Treat. 126, 453–461 (2011).
Cigler, T. et al. A placebo-controlled trial examining the effects of letrozole on mammographic breast density and bone and lipid metabolism [abstract]. Breast Cancer Res. Treat. 106 (Suppl.), a2082 (2007).
Vachon, C. M. et al. Longitudinal trends in mammographic percent density and breast cancer risk. Cancer Epidemiol. Biomarkers Prev. 16, 921–928 (2007).
Vachon, C. M. et al. Mammographic breast density response to aromatase inhibition. Clin. Cancer Res. 19, 2144–2153 (2013).
Vidi, P. A., Leary, J. F. & Lelièvre, S. A. Building risk-on-a-chip models to improve breast cancer risk assessment and prevention. Integr. Biol. (Camb.) 5, 1110–1118 (2013).
Wang, W. & Srivastava, S. Strategic approach to validating methylated genes as biomarkers for breast cancer. Cancer Prev. Res. (Phila.) 3, 16–24 (2010).
Fabian, C. J. et al. Short-term breast cancer prediction by random periareolar fine-needle aspiration cytology and the Gail risk model. J. Natl Cancer Inst. 92, 1217–1227 (2000).
Shaaban, A. M., Sloane, J. P., West, C. R. & Foster, C. S. Breast cancer risk in usual ductal hyperplasia is defined by estrogen receptor-alpha and Ki-67 expression. Am. J. Pathol. 160, 597–604 (2002).
King, B. L. & Love, S. M. The intraductal approach to the breast: raison d'être. Breast Cancer Res. 8, 206 (2006).
Chaiwun, B. & Thorner, P. Fine needle aspiration for evaluation of breast masses. Curr. Opin. Obstet. Gynecol. 19, 48–55 (2007).
Stomper, P. C., Budnick, R. M. & Stewart, C. C. Use of specimen mammography-guided FNA (fine-needle aspirates) for flow cytometric multiple marker analysis and immunophenotyping in breast cancer. Cytometry 42, 165–173 (2000).
Bondeson, L. & Lindholm, K. Prediction of invasiveness by aspiration cytology applied to nonpalpable breast carcinoma and tested in 300 cases. Diagn. Cytopathol. 17, 315–320 (1997).
Akhtar, M., Bakry, M., al-Jeaid, A. S. & McClintock, J. A. Electron. microscopy of fine-needle aspiration biopsy specimens: a brief review. Diagn. Cytopathol. 8, 278–282 (1992).
Henry-Stanley, M. J. & Stanley, M. W. Processing of needle rinse material from fine-needle aspirations rarely detects malignancy not identified in smears. Diagn. Cytopathol. 8, 538–540 (1992).
Shidham, V. B., Pandit, A. W., Rao, R. N., Basir, Z. & Shidham, A. Tissue Harvester with Functional Valve (THFV): Shidham's device for reproducibly higher specimen yield by fine needle aspiration biopsy with easy to perform steps. BMC Clin. Pathol. 7, 2 (2007).
Yang, J. H. et al. Effect of core-needle biopsy vs fine-needle aspiration on pathologic measurement of tumor size in breast cancer. Arch. Surg. 140, 125–128 (2005).
Fabian, C. J., Kimler, B. F., Mayo, M. S. & Khan, S. A. Breast-tissue sampling for risk assessment and prevention. Endocr. Relat. Cancer 12, 185–213 (2005).
Easton, D. F. et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature 447, 1087–1093 (2007).
Stacey, S. N. et al. Common variants on chromosome 5p12 confer susceptibility to estrogen receptor-positive breast cancer. Nat. Genet. 40, 703–706 (2008).
Stacey, S. N. et al. Common variants on chromosomes 2q35 and 16q12 confer susceptibility to estrogen receptor-positive breast cancer. Nat. Genet. 39, 865–869 (2007).
Ingle, J. N. et al. Selective estrogen receptor modulators and pharmacogenomic variation in ZNF423 regulation of BRCA1 expression: individualized breast cancer prevention. Cancer Discov. 3, 812–825 (2013).
Darabi, H. et al. Breast cancer risk prediction and individualised screening based on common genetic variation and breast density measurement. Breast Cancer Res. 14, R25 (2012).
Dite, G. S. et al. Using SNP genotypes to improve the discrimination of a simple breast cancer risk prediction model. Breast Cancer Res. Treat. 139, 887–896 (2013).
Kelloff, G. J. et al. Risk biomarkers and current strategies for cancer chemoprevention. J. Cell. Biochem. 25 (Suppl.), 1–14 (1996).
Boone, C. W., Bacus, J. W., Bacus, J. V., Steele, V. E. & Kelloff, G. J. Properties of intraepithelial neoplasia relevant to cancer chemoprevention and to the development of surrogate end points for clinical trials. Proc. Soc. Exp. Biol. Med. 216, 151–165 (1997).
Dunn, B. K., Jegalian, K. & Greenwald, P. Biomarkers for early detection and as surrogate endpoints in cancer prevention trials: issues and opportunities. Recent Results Cancer Res. 188, 21–47 (2011).
Rutqvist, L. E. et al. Contralateral primary tumors in breast cancer patients in a randomized trial of adjuvant tamoxifen therapy. J. Natl Cancer Inst. 83, 1299–1306 (1991).
Veronesi, U. et al. Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomised trial among hysterectomised women. Italian Tamoxifen Prevention Study. Lancet 352, 93–97 (1998).
Martino, S. et al. Effect of raloxifene on incidence of invasive breast cancer in postmenopausal women stratified by baseline serum estradiol: results of the Continuing Outcomes Relevant to Evista (CORE) trial [abstract]. Breast Cancer Res. Treat. 88 (Suppl.), a22 (2004).
Powles, T. J., Ashley, S., Tidy, A., Smith, I. E. & Dowsett, M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J. Natl Cancer Inst. 99, 283–290 (2007).
Fisher, B. et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J. Natl Cancer Inst. 97, 1652–1662 (2005).
Veronesi, U. et al. Tamoxifen for the prevention of breast cancer: late results of the Italian Randomized Tamoxifen Prevention Trial among women with hysterectomy. J. Natl Cancer Inst. 99, 727–737 (2007).
Barrett-Connor, E. et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N. Engl. J. Med. 355, 125–137 (2006).
Vogel, V. G. et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev. Res. (Phila.) 3, 696–706 (2010).
Cummings, S. R. et al. Lasofoxifene in postmenopausal women with osteoporosis. N. Engl. J. Med. 362, 686–696 (2010).
Cummings, S. R. et al. Arzoxifene for prevention of fractures and invasive breast cancer in postmenopausal women. J. Bone Miner. Res. 26, 397–404 (2011).
Meyskens, F. L. Jr et al. Regulatory approval of cancer risk-reducing (chemopreventive) drugs: moving what we have learned into the clinic. Cancer Prev. Res. (Phila.) 4, 311–323 (2011).
Familial breast cancer: classification and care of people at risk of familial breast cancer and management of breast cancer and related risks in people with a family history of breast cancer. NICE clinical guideline 164. National Institute for Health and Care Excellence [online], (2013).
Buzdar, A. The ATAC ('Arimidex', Tamoxifen, Alone or in Combination) trial in postmenopausal women with early breast cancer—updated efficacy results based on a median follow-up of 47 months. Breast Cancer Res. Treat. 77, 295 (2003).
van de Velde, C. J. et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trial. Lancet 377, 321–331 (2011).
Thuerlimann, B. et al. BIG 1–98: Randomized double-blind phase III study to evaluate letrozole (L) vs. tamoxifen (T) as adjuvant endocrine therapy for postmenopausal women with receptor-positive breast cancer [abstract]. J. Clin. Oncol. 23 (Suppl.), a511 (2005).
Kraus, S., Naumov, I. & Arber, N. COX-2 active agents in the chemoprevention of colorectal cancer. Recent Results Cancer Res. 191, 95–103 (2013).
Lee, C. S., McNamara, D. & O'Morain, C. A. Aspirin as a chemoprevention agent for colorectal cancer. Curr. Drug Metab. 13, 1313–1322 (2012).
Brophy, J. M. Cardiovascular effects of cyclooxygenase-2 inhibitors. Curr. Opin. Gastroenterol. 23, 617–624 (2007).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2005).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
Lochhead, P. & Chan, A. T. Statins and colorectal cancer. Clin. Gastroenterol. Hepatol. 11, 109–118 (2013).
Bruno, A., Dovizio, M., Tacconelli, S. & Patrignani, P. Mechanisms of the antitumoural effects of aspirin in the gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 26, e1–e13 (2012).
Lin, H. C. et al. Effects of metformin dose on cancer risk reduction in patients with type 2 diabetes mellitus: a 6-Year follow-up study. Pharmacotherapy http://dx.doi.org/10.1002/phar.1334.
Dowsett, M. et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J. Clin. Oncol. 28, 509–518 (2010).
van Nes, J. G. et al. Quality of life in relation to tamoxifen or exemestane treatment in postmenopausal breast cancer patients: a Tamoxifen Exemestane Adjuvant Multinational (TEAM) Trial side study. Breast Cancer Res. Treat. 134, 267–276 (2012).
Goss, P. E. et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J. Natl Cancer Inst. 97, 1262–1271 (2005).
Davies, C. et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381, 805–816 (2013).
Goss, P. E., Muss, H. B., Ingle, J. N., Whelan, T. J. & Wu, M. Extended adjuvant endocrine therapy in breast cancer: current status and future directions. Clin. Breast Cancer 8, 411–417 (2008).
Young, R. J. & Coleman, R. E. Zoledronic acid to prevent and treat cancer metastasis: new prospects for an old drug. Future Oncol. 9, 633–643 (2013).
Gnant, M. et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N. Engl. J. Med. 360, 679–691 (2009).
Ford, J. A. et al. Denosumab for treatment of bone metastases secondary to solid tumours: systematic review and network meta-analysis. Eur. J. Cancer 49, 416–430 (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Wiedermann, U., Davis, A. B. & Zielinski, C. C. Vaccination for the prevention and treatment of breast cancer with special focus on Her-2/neu peptide vaccines. Breast Cancer Res. Treat. 138, 1–12 (2013).
Glimelius, B. & Lahn, M. Window-of-opportunity trials to evaluate clinical activity of new molecular entities in oncology. Ann. Oncol. 22, 1717–1725 (2011).
Kummar, S. et al. Phase 0 clinical trials: conceptions and misconceptions. Cancer J. 14, 133–137 (2008).
Coombes, R. C. et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N. Engl. J. Med. 350, 1081–1092 (2004).
Napoli, M. et al. Letter to FDA opposing use of surrogate endpoints for approval of cancer prevention drugs. Center for Medical Consumers [online], (2003).
US National Institutes of Health. ClinicalTrials.gov [online], (2013).
Baker, S. G. & Kramer, B. S. Surrogate endpoint analysis: an exercise in extrapolation. J. Natl Cancer Inst. 105, 316–320 (2013).
Ropka, M. E., Keim, J. & Philbrick, J. T. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J. Clin. Oncol. 28, 3090–3095 (2010).
Visvanathan, K. et al. American Society of Clinical Oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J. Clin. Oncol. 27, 3235–3258 (2009).
Waters, E. A., Cronin, K. A., Graubard, B. I., Han, P. K. & Freedman, A. N. Prevalence of tamoxifen use for breast cancer chemoprevention among U. S. women. Cancer Epidemiol. Biomarkers Prev. 19, 443–446 (2010).
Lippman, S. M. The dilemma and promise of cancer chemoprevention. Nat. Clin. Pract. Oncol. 3, 523 (2006).
Armstrong, K., Quistberg, D. A., Micco, E., Domchek, S. & Guerra, C. Prescription of tamoxifen for breast cancer prevention by primary care physicians. Arch. Intern. Med. 166, 2260–2265 (2006).
Rockhill, B., Spiegelman, D., Byrne, C., Hunter, D. J. & Colditz, G. A. Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J. Natl Cancer Inst. 93, 358–366 (2001).
Foskett, J. Constructing “high risk women”: the development and standardization of a breast cancer risk assessment tool. Sci. Technol. Human Values 29, 291–313 (2004).
Kaplan, C. P. et al. Breast cancer risk reduction options: awareness, discussion, and use among women from four ethnic groups. Cancer Epidemiol. Biomarkers Prev. 15, 162–166 (2006).
Savage, L. Researchers wonder why high-risk women are not taking chemoprevention drugs. J. Natl Cancer Inst. 99, 913–914 (2007).
Hux, J. E., Levinton, C. M. & Naylor, C. D. Prescribing propensity: influence of life-expectancy gains and drug costs. J. Gen. Intern. Med. 9, 195–201 (1994).
Lipkus, I. M., Klein, W. M. & Rimer, B. K. Communicating breast cancer risks to women using different formats. Cancer Epidemiol. Biomarkers Prev. 10, 895–898 (2001).
Malenka, D. J., Baron, J. A., Johansen, S., Wahrenberger, J. W. & Ross, J. M. The framing effect of relative and absolute risk. J. Gen. Intern. Med. 8, 543–548 (1993).
Mazur, D. J. & Hickam, D. H. Patients' and physicians' interpretations of graphic data displays. Med. Decis. Making 13, 59–63 (1993).
McGettigan, P., Sly, K., O'Connell, D., Hill, S. & Henry, D. The effects of information framing on the practices of physicians. J. Gen. Intern. Med. 14, 633–642 (1999).
Tversky, A. & Kahneman, D. Judgment under uncertainty: heuristics and biases. Science 185, 1124–1131 (1974).
Woloshin, S., Schwartz, L. M., Black, W. C. & Welch, H. G. Women's perceptions of breast cancer risk: how you ask matters. Med. Decis. Making 19, 221–229 (1999).
Ancker, J. S., Senathirajah, Y., Kukafka, R. & Starren, J. B. Design features of graphs in health risk communication: a systematic review. J. Am. Med. Inform. Assoc. 13, 608–618 (2006).
Edwards, A. Communicating risks through analogies. BMJ 327, 749 (2003).
Hibbard, J. H. & Peters, E. Supporting informed consumer health care decisions: data presentation approaches that facilitate the use of information in choice. Annu. Rev. Public Health 24, 413–433 (2003).
Lipkus, I. M. & Hollands, J. G. The visual communication of risk. J. Natl Cancer Inst. Monogr. 1999, 149–163 (1999).
Stone, E. R. et al. Foreground: background salience: explaining the effects of graphical displays on risk avoidance. Organ. Behav. Hum. Decis. Process. 90, 19–36 (2003).
Bober, S. L., Hoke, L. A., Duda, R. B., Regan, M. M. & Tung, N. M. Decision-making about tamoxifen in women at high risk for breast cancer: clinical and psychological factors. J. Clin. Oncol. 22, 4951–4957 (2004).
Goldenberg, V. K. et al. Atypia in random periareolar fine-needle aspiration affects the decision of women at high risk to take tamoxifen for breast cancer chemoprevention. Cancer Epidemiol. Biomarkers Prev. 16, 1032–1034 (2007).
Author information
Authors and Affiliations
Contributions
K. Strasser-Weippl researched data for the manuscript. Both authors made a substantial contribution to discussion of the article content, wrote the manuscript and edited it before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Strasser-Weippl, K., Goss, P. Suitable trial designs and cohorts for preventive breast cancer agents. Nat Rev Clin Oncol 10, 677–687 (2013). https://doi.org/10.1038/nrclinonc.2013.174
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2013.174
This article is cited by
-
Simvastatin treatment varies the radiation response of human breast cells in 2D or 3D culture
Investigational New Drugs (2021)
-
Physician and Patient Barriers to Breast Cancer Preventive Therapy
Current Breast Cancer Reports (2016)
-
Risk determination and prevention of breast cancer
Breast Cancer Research (2014)