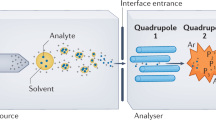
Key Points
-
Mass spectrometry has become an indispensable analytical tool in all types of drug discovery applications.
-
Development of two ionization techniques, electrospray (ESI) and matrix assisted laser desorption/ionization (MALDI), has revolutionized the applicability of mass spectrometry to almost any biological molecule.
-
Many types of mass analyzers can be used in drug discovery applications, but the majority of the work is done with quadrupoles, quadrupole ion traps, and time-of-flight mass spectrometers. Fourier-transform ion-cyclotron resonance (FT-ICR) mass spectrometry is playing an increasingly important role as a mass analyzer.
-
Tandem mass spectrometry (MS/MS) is a crucial technique in many applications related to drug discovery. MS/MS involves multiple stages of mass analysis.
-
Quadrupole ion traps and FT-ICR mass spectrometers perform MS/MS by separating the stages in time. Other combinations of analyzers such as quadrupole/time-of-flight and multiple quadrupole mass spectrometers perform MS/MS using sequential analyzers.
Abstract
Enormous advances in our understanding of the chemistry underlying life processes have identified new targets for therapeutic agents. The discovery of effective therapeutics to address these targets is often accomplished through parallel synthetic and screening efforts. In almost all cases, what has enabled target identification and allowed parallel approaches to drug discovery to be effective are the development of either new analytical tools or the improvement of currently existing ones. Among these tools, mass spectrometry has evolved to become an irreplaceable technique in the analysis of biologically related molecules. This article will guide researchers in drug discovery through the basic principles of mass spectrometry.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lee, M. S. & Kerns, E. H. LC/MS applications in drug development. Mass Spectrom. Rev. 18, 187–279 (1999).
Cheng, X. & Hochlowski, J. Current application of mass spectrometry to combinatorial chemistry. Anal. Chem. 74, 2679–2690 (2002).
Triolo, A., Altamura, M., Cardinali, F., Sisto, A. & Maggi, C. A. Mass spectrometry and combinatorial chemistry: a short outline. J. Mass Spectrom. 36, 1249–1259 (2001).
Shin, Y. G. & Breemen, R. B. v. Analysis and screening of combinatorial libraries using mass spectrometry. Biopharm. Drug Dispos. 22, 353–372 (2001).
Kassel, D. B. Combinatorial chemistry and mass spectrometry in the 21st century drug discovery laboratory. Chem. Rev. 101, 255–267 (2001).
Yamashita, M. & Fenn, J. B. Electrospray ion source. another variation of the free-jet theme. J. Phys. Chem. 88, 4451–4459 (1984). The initial publication in the development of electrospray as a useful ionization technique for mass spectrometry.
Karas, M., Bachmann, D., Bahr, U. & Hillenkamp, F. Matrix-assisted ultraviolet laser desorption of non-volatile compounds. Int. J. Mass Spectrom. Ion Processes 78, 53–68 (1987). Seminal publication describing the use of a matrix to enhance the ionization efficiency of non-volatile compounds — this method of laser/desorption ionization is the most commonly used today.
Horning, E. C., Horning, M. G., Carroll, D. I., Dzidic, I. & Stillwell, R. N. New Picogram detection system based on a mass spectrometer with an external ionization source at atmospheric pressure. Anal. Chem. 45, 936–943 (1973).
Chen, R. et al. Trapping, detection, and mass determination of coliphage T4 DNA Ions by electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Anal. Chem. 67, 1159–1163 (1995).
Smith, R. D., Cheng, X., Bruce, J. E., Hofstadler, S. A. & Anderson, G. A. Trapping, detection, and reaction of very large single molecular ions by mass spectrometry. Nature 369, 137–139 (1994).
Smith, R. D., Bruce, J. E., Wu, Q. & Lei, Q. P. New mass spectrometric methods for the study of noncovalent associations of biopolymers. Chem. Soc. Rev. 26, 191–202 (1997). A good review of how mass spectrometry is used to study non-covalent complexes of proteins, DNA, DNA–drug interactions and enzyme–inhibitor interactions.
Suizdak, G. et al. Mass spectrometry and viral analysis. Chem. Biol. 3, 45–48 (1996).
Berggren, W. T., Westphall, M. S. & Smith, L. M. Single-pulse nanoelectrospray ionization. Anal. Chem. 74, 3443–3448 (2002).
Lu, Y., Zhou, F., Shui, W., Bian, L., Guo, Y. & Yang, P. Pulsed Electrospray for mass spectrometry. Anal. Chem. 73, 4748–4753 (2001).
Siegel, M. M., Tabei, K., Lambert, F., Candela, L. & Zoltan, B. Evaluation of a dual electrospray ionization/atmospheric pressure chemical ionization source at low flow rates (50μl/min) for analysis of both highly and weakly polar compounds. J. Am. Soc. Mass Spectrom. 9, 1196–1203 (1998).
Tanaka, K. et al. Protein and polymer analyses up to m/z 100000 by laser ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2, 151–153 (1988).
Laiko, V. V., Baldwin, M. A. & Burlingame, A. L. Atmospheric pressure matrix-assisted laser desorption/ionization mass spectrometry. Anal. Chem. 72, 652–657 (2000).
Laiko, V. V., Moyer, S. C. & Cotter, R. J. Atmospheric pressure MALDI/Ion trap mass spectrometry. Anal. Chem. 72, 5239–5243 (2000).
Lay, J. O. MALDI–TOF Mass spectrometry of bacteria. Mass Spectrom. Rev. 20, 172–194 (2001).
Fenselau, C. & Demirev, P. A. Characterization of intact microorganisms by MALDI mass spectrometry. Mass Spectrom. Rev. 20, 157–171 (2001).
Thomas, J. J., Shen, Z., Crowell, J. E., Finn, M. G. & Siuzdak, G. Desorption/ionization on silicon (DIOS): a diverse mass spectrometry platform for protein characterization. Proc. Natl Acad. Sci. USA 98, 4932–4937 (2001).
Shen, Z. et al. Porous silicon as a versatile platform for laser desorption/ionization mass spectrometry. Anal. Chem. 73, 612–619 (2001).
Weickhardt, C., Moritz, F. & Grotemeyer, J. Time-of-flight mass spectrometry: state-of the-art in chemical analysis and molecular science. Mass Spectrom. Rev. 15, 139–162 (1996).
Wiley, W. C. & McLaren, I. H. Time of flight mass spectrometer with improved resolution. Rev. Sci. Instr. 26, 1150–1157 (1955).
Stafford, G. C. J., Kelley, P. E., Syka, J. E. P., Reynolds, W. E. & Todd, J. F. J. Recent improvements in and analytical applications of advanced ion trap technology. Int. J. Mass Spectrom. Ion Processes 60, 85–98 (1984). A description of important improvements to the quadrupole ion trap mass spectrometer that eventually led to its wide use today.
Schwartz, J. C., Syka, J. E. P. & Jardine, I. High resolution on a quadrupole ion trap mass spectrometer. J. Am. Soc. Mass Spectrom. 2, 198–204 (1991).
Goeringer, D. E., Whitten, W. B., Ramsey, J. M., McLuckey, S. A. & Glish, G. L. Theory of high-resolution mass spectrometry achieved via resonance ejection in the quadrupole ion trap. Anal. Chem. 64, 1434–1439 (1992).
Kaiser, R. E. J., Louris, J. N., Amy, J. W. & Cooks, R. G. Extending the mass range of the quadrupole ion trap using axial modulation. Rapid Commun. Mass Spectrom. 3, 225–229 (1989).
Marshall, A. G. & Comisarow, M. Fourier transform ion cyclotron resonance [FT–ICR] spectroscopy. Chem. Phys. Lett. 25, 282–283 (1974). The first demonstration of the use of Fourier-transform ion-cyclotron resonance for mass analysis in ion-cyclotron resonance mass spectrometers.
Marshall, A. G., Hendrickson, C. L. & Jackson, G. S. Fourier transform ion cyclotron resonance mass spectrometry: a primer. Mass Spectrom. Rev. 17, 1–35 (1998).
Busch, K. L., Glish, G. L. & McLuckey, S. A. Mass Spectrometry/Mass Spectrometry: Techniques and Applications of Tandem Mass Spectrometry (VCH New York, 1988).
Carr, S. A., Huddleston, M. J. & Bean, M. E. Selective identification and differentiation of N- and O-linked oligosaccharides in glycoproteins by liquid chromatography–mass spectrometry. Protein Sci. 2, 183–196 (1993).
Schlosser, A., Pipkorn, R., Bossemeyer, D. & Lehmann, W. D. Analysis of protein phosphorylation by a combination of elastase digestion and neutral loss tandem mass spectrometry. Anal. Chem. 73, 170–176 (2001).
McLuckey, S. A., Principles of collisional activation in analytical mass spectrometry. J. Am. Soc. Mass Spectrom. 3, 599–614 (1992).
Dongré, A. R., Somogyi, A. & Wysocki, V. H. Surface-induced dissociation: an effective tool to probe structure, energetics and fragmentation mechanisms of protonated peptides. J. Mass Spectrom. 31, 339–350 (1996).
Mabud, M. A., Dekrey, M. J. & Cooks, R. G. Surface-induced dissociation of molecular ions. Int. J. Mass Spectrom. Ion Processes 67, 285–294 (1985).
Hunt, D. F., Shabanowitz, J. & Yates, J. R. Peptide sequence analysis by laser photodissociation Fourier transform mass spectrometry. J. Chem. Soc. Chem. Commun. 548–550 (1987).
Tecklenburg, R. E., Miller, M. N. & Russell, D. H. Laser ion beam photodissociation studies of model amino acids and peptides. J. Am. Chem. Soc. 111, 1161–1171 (1989).
Goolsby, B. J. & Brodbelt, J. S. Characterization of β-lactams by photodissociation and collision-activated dissociation in a quadrupole ion trap. J. Mass Spectrom. 33, 705–712 (1998).
Payne, A. H. & Glish, G. L. Thermally assisted infrared multiphoton photodissociation in a quadrupole ion trap. Anal. Chem. 73, 3542–3548 (2001).
Little, D. P., Speir, J. P., Senko, M. W., O'Connor, P. B. & McLafferty, F. W. Infrared multiphoton dissociation of large multiply charged ions for biomolecule sequencing. Anal. Chem. 66, 2809–2815 (1994).
Glish, G. L. Multiple stage mass spectrometry: the next generation tandem mass spectrometry experiment. Analyst 119, 533–537 (1994).
Kondrat, R. W. & Cooks, R. G. Direct analysis of mixtures by mass spectrometry. Anal. Chem. 50, 81A–92A (1978).
Yost, R. A. & Enke, C. G. Selected ion fragmentation with a tandem quadrupole mass spectrometer. J. Am. Chem. Soc. 100, 2274–2275 (1978). The first demonstration of a triple quadrupole mass spectrometer and its advantages for structural analysis by tandem mass spectrometry.
Glish, G. L. & Goeringer, D. E. Tandem quadrupole/time-of-flight instrument for mass spectrometry/mass spectrometry. Anal. Chem. 56, 2291–2295 (1984). The first tandem quadrupole/time of flight mass spectrometer, developed for high-speed and high-sensitivity MS/MS.
Wan, K. X., Gross, M. L. & Shibue, T. Gas-phase stability of double-stranded oligodeoxynucleotides and their noncovalent complexes with DNA-binding drugs as revealed by collisional activation in an ion trap. J. Am. Soc. Mass Spectrom. 11, 450–457 (2000).
Gale, D. C., Goodlett, D. R., Light-Wahl, K. J. & Smith, R. D. Observation of duplex DNA–drug noncovalent complexes by electrospray ionization mass spectrometry. J. Am. Chem. Soc. 116, 6027–6028 (1994).
Kapur, A., Beck, J. L. & Sheil, M. M. Observation of daunomycin and nogalamycin complexes with duplex DNA using electrospray ionisation mass spectrometry. Rapid Commun. Mass Spectrom. 13, 2489–2497 (1999).
Gabelica, V. DePauw, E. & Rosu, F. Interaction between antitumor drugs and a double-stranded oligonucleotide studied by electrospray ionization mass spectrometry. J. Mass Spectrom. 34, 1328–1337 (1999).
Gupta, R., Kapur, A., Beck, J. L. & Sheil, M. M. Positive ion electrospray ionization mass spectrometry of double-stranded DNA/drug complexes. Rapid Commun. Mass Spectrom. 15, 2472–2480 (2001).
David, W. M., Brodbelt, J., Kerwin, S. M. & Thomas, P. W. Investigation of quadruplex oligonucleotide–drug interactions by electrospray ionization mass spectrometry. Anal. Chem. 74, 2029–2033 (2002).
Rosu, F., Gabelica, V., Houssier, C. & DePauw, E. Determination of affinity, stoichiometry and sequence selectivity of minor groove binder complexes with double-stranded oligodeoxynucleotides by electrospray ionization mass spectrometry. Nucleic Acids Res. 30, e82 (2002).
Hofstadler, S. A. et al. Multiplexed screening of neutral mass-tagged RNA targets against ligand libraries with electrospray ionization FTICR MS: a paradigm for high-throughput affinity screening. Anal. Chem. 71, 3436–3440 (1999).
Masselon, C., Anderson, G. A., Harkewicz, R., Bruce, J. E., Pasa-Tolic, L. & Smith, R. D. Accurate mass multiplexed tandem mass spectrometry for high-throughput polypeptide identification from mixtures. Anal. Chem. 72, 1918–1924 (2000).
Wabnitz, P. A. & Loo, J. A. Drug screening of pharmaceutical discovery compounds by micro-size exclusion chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 16, 85–91 (2002).
Moy, F. J. et al. MS/NMR: A structure-based approach for discovering protein ligands and for drug design by coupling size exclusion chromatography. Mass spectrometry, and nuclear magnetic resonance spectroscopy. Anal. Chem. 73, 571–581 (2001).
Siegel, M. M., Tabei, K., Bebernitz, G. A. & Baum, E. Z. Rapid methods for screening low molecular mass compounds non-covalently bound to proteins using size exclusion and mass spectrometry applied to inhibitors of human cytomegalovirus protease. J. Mass Spectrom. 33, 264–273 (1998).
Kaltashov, I. A. & Eyles, S. J. Studies of biomolecular conformations and conformational dynamics by mass spectrometry. Mass Spectrom. Rev. 21, 37–71 (2002).
Smith, D. L., Deng, Y. & Zhang, Z. Probing the non-covalent structure of proteins by amide hydrogen exchange and mass spectrometry. J. Mass Spectrom. 32, 135–146 (1997).
Engen, J. R. & Smith, D. L. Investigating protein structure and dynamics by hydrogen exchange MS. Anal. Chem. 73, 256A–265A (2001). A good review of the use of mass spectrometry with hydrogen exchange to probe structure and dynamics.
Deng, Y., Pan, H. & Smith, D. L. Selective isotope labeling demonstrates that hydrogen exchange at individual peptide amide linkages can be determined by collision-induced dissociation mass spectrometry. J. Am. Chem. Soc. 121, 1966–1967 (1999).
Eyles, S. J., Speir, J. P., Kruppa, G. H., Gierasch, L. M. & Kaltashov, I. A. Protein conformational stability probed by Fourier transform ion cyclotron resonance mass spectrometry. J. Am. Chem. Soc. 122, 495–500 (2000).
Kaltashov, I. A. & Eyles, S. J. Crossing the phase boundary to study protein dynamics and function: combination of amide hydrogen exchange in solution and ion fragmentation in the gas phase. J. Mass Spectrom. 37, 557–565 (2002).
Aebersold, R. & Goodlett, D. R. Mass spectrometry in proteomics. Chem. Rev. 101, 269–295 (2001).
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
FURTHER INFORMATION
International Mass Spectrometry Society
American Society for Mass Spectrometry
Enclyclopedia of Life Sciences
Glossary
- ANALYTE
-
The substance being analyzed.
- PROTONATION
-
The addition of one or more protons to a compound so that the net charge of the compound is positive.
- DEPROTONATION
-
The removal of one or more protons from a compound so that the net charge of the compound is negative.
- KINETIC ENERGY
-
The energy associated with a substance because of its translational motion; equal to one-half its mass times the square of its velocity.
- INTERNAL ENERGY
-
The energy associated with the movement of the atoms and electrons in a chemical compound relative to one another; the increase in the internal energy of a compound leads to an increase in its temperature and in some cases dissociation.
- STOICHIOMETRY
-
The quantitative relationship between the reactants and products in a chemical reaction.
Rights and permissions
About this article
Cite this article
Glish, G., Vachet, R. The basics of mass spectrometry in the twenty-first century. Nat Rev Drug Discov 2, 140–150 (2003). https://doi.org/10.1038/nrd1011
Issue Date:
DOI: https://doi.org/10.1038/nrd1011
This article is cited by
-
Mass spectra prediction with structural motif-based graph neural networks
Scientific Reports (2024)
-
High-resolution separation of bioisomers using ion cloud profiling
Nature Communications (2023)
-
In situ droplet-based on-tissue chemical derivatization for lipid isomer characterization using LESA
Analytical and Bioanalytical Chemistry (2023)
-
Growing single crystals of two-dimensional covalent organic frameworks enabled by intermediate tracing study
Nature Communications (2022)
-
Emerging proteomic approaches to identify the underlying pathophysiology of neurodevelopmental and neurodegenerative disorders
Molecular Autism (2020)