Key Points
-
Allergic rhinitis is a common manifestation of atopy whose prevalence world wide is increasing in association with a Western-type lifestyle.
-
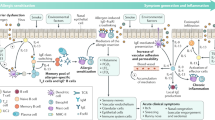
Pathogenesis involves the selective upregulation of Th2 type lymphocytes in the nasal mucosal driven by exposure to seasonal or perennial aeroallergens.
-
Activation of Th2 cells leads to the release of cytokines that induce isotype switching of B cells to IgE production and the recruitment and activation of mast cells and circulating secondary effector leukocytes.
-
While topical corticosteroids and H1-antihistamines are effective treatments for allergic rhinitis, incomplete clinical responses and concern over side effects has led to a search for new ways offer intervening.
-
Improved ways of stabilising mast cells, the identification of novel mediators and their receptors and an understanding of intracellular signalling pathways of effector cells offer new opportunities for therapeutic intervention.
-
Blockade of IgE, the activation signal for allergic rhinitis, using monoclonal antibodies or vaccine strategies are being introduced for the treatment of asthma and accompanying atopic disorders which includes allergic rhinitis.
-
New forms or allergen-specific immunotherapy (SIT and SLIT) and ways to improve efficacy and reduce side effects include the use of recombinant mutated allergens, peptides and CpG DNA oligonucleotides (Immunostimulatory Sequences-ISS)
-
An understanding of the molecular mechanisms of leukocyte adhesion, migration and activation is leading to therapeutics including adhesion molecular antagonists, chemokine inhibitors and receptor antagonists and agents that inhibit mediator release including Type 4 PDE inhibitors.
-
In the long term, modification of the mechanisms underlying the increased expression of atopy is likely to have a greater overall impact on allergic rhinitis than any specific therapy targeting the nasal mucosa.
Abstract
Allergic rhinitis is an inflammatory disorder of the nasal mucosa, mediated by TH2 lymphocytes, which is linked to atopy and whose prevalence is increasing in association with a Western lifestyle. The production of allergen-specific IgE, activation of mucosal mast cells and the recruitment and activation of effector leukocytes provides potential therapeutic targets, including selective inhibition of cytokines, adhesion molecules and signalling pathways. Blockade of IgE, using monoclonal antibodies and vaccine strategies, is a new approach for interrupting the allergic cascade, whereas the use of recombinant mutated allergens, peptides and DNA oligonucleotides will lead to improved efficacy and reduced side effects of immunotherapy to induce tolerance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Skoner, D. P. Allergic rhinitis: definition, epidemiology, pathophysiology, detection and diagnosis. J. Allergy Clin. Immunol. 108, S2–S8 (2001).
Law, A. W., Reed, S. D., Sundy, J. S. & Schulman, K. A. Direct costs of allergic rhinitis in the United States: estimates from the 1996 Medical Expenditure Panel Survey. J. Allergy Clin. Immunol. 111, 296–300 (2003).
Sullivan, S. D. & Weiss, K. B. Health economics of asthma and rhinitis. II. Assessing the value of interventions. J. Allergy Clin. Immunol. 107, 203–210 (2001).
Crystal-Peters, J., Neslusan, C., Crown, W. H. & Torres, A. Treating allergic rhinitis in patients with comorbid asthma: the risk of asthma-related hospitalizations and emergency department visits. J. Allergy Clin. Immunol. 109, 57–62 (2002). Allergic rhinitis carries a high co-morbidity with other atopic disorders including asthma, as highlighted by the WHO ARIA report.
Cookson, W. Genetics and genomics of asthma and allergic diseases. Immunol. Rev. 190, 195–206 (2002).
Umetsu, D. T., McIntire, J. J., Akbari, O., Macaubas, C. & De Kruyff, R. H. Asthma: an epidemic of dysregulated immunity. Nature Immunol. 3, 715–720 (2002). This timely review emphasizes the importance of T-cell tolerance in protecting against atopic disease and describes how this can be harnessed for new treatments.
Matricardi, P. M., Rosmini, F., Paneta, V., Ferrigao, L. & Bonini, S. Hayfever and asthma in relation to markers of infection in the United States. J. Allergy Clin. Immunol. 110, 381–387 (2002).
Kay, A. B. Allergy and allergic diseases. N. Engl. J. Med. 344, 109–113 (2001).
Pullerits, T., Praks, L., Ristioja, V. & Lottval, J. Comparison of a nasal glucocorticoid, antileukotriene and a combination of antileukotriene and antihistamine in the treatment of seasonal allergic rhinitis. J. Allergy Clin. Immunol. 109, 949–955 (2002).
Oppenheimer, J. J. & Casale, T. B. Next generation antihsitamines: therapeutic rationale, accomplishments and advances. Expert Opin. Investig. Drugs. 11, 807–817 (2002).
Baroody, F. M. & Naclerio, R. M. Antiallergic effects of H1-receptor antagonists. Allergy 55 (Suppl 64), 17–27 (2000).
Hofstra, C. L., Desai, P. J., Thurmond, R. L. & Fung-Leung, W. P. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J. Pharmacol. Exp. Ther. 305, 1212–1221 (2003). The identification of histamine H 4 receptors has provided a new target for the design of antihistamine drugs for the treatment of allergic inflammation.
Higashi, N., Taniguchi, M., Mita, H., Ishii, T. & Akiyama, K. Nasal blockage and urinary leukotriene E4 concentration in patients with seasonal allergic rhinitis. Allergy 58, 476–480 (2003).
Philip, G. et al. Montelukast for treating seasonal allergic rhinitis: a random double-blind, placebo-controlled trial performed in the sprin. Clin. Exp. Allergy 32, 1020–1028 (2002).
Topuz, B. & Ogmen, G. G. Monelukast as an adjuvant to mainstay therapies in patients seasonal allergic rhinitis. Clin. Exp. Allergy 33, 823–826 (2003).
Nayak, A. S., Philip, G., Lu, S., Malice, M. P. & Reiss, T. F. Efficacy and tolerability of montelukast alone or in combination with loratadine in seasonal allergic rhinitis: a multicentre, randomized, double-blind, placebo-controlled trial performed in the fall. Ann. Allergy Asthma Immunol. 88, 592–600 (2002).
Evans, J. F. Cysteinyl leukotriene receptors. Prostaglandins Other Lipid Mediat. 68–69, 587–597 (2002).
Arimura, A. et al. Prevention of allergic inflammation by a novel prostaglandin receptor antagonist, S-5751. J. Pharmacol. Exp. Ther. 298, 411–419 (2001).
Sugimoto, H. et al. An orally bioavailable small molecule antagonist of CRTH2, ramatroban (BAY u3405), inhibits prostaglandin D2-induced eosinophil migration in vitro. J. Pharmacol. Exp. Ther. 305, 347–352 (2003). The mast cell eicosanoid prostaglandin D 2 has created a new target for therapy by blockade of the DP 1 and the newly recognized DP 2 receptors.
Vanderslice, P. et al. Human mast cell tryptase: Multiple cDNAs and genes reveal a multigene serine protease family. Proc. Natl Acad. Sci. USA 87, 3811–3815 (1990).
Wong, G. W. et al. Biochemical and functional characaterization of human transmembrane tryptase (TMT), tryptase-γ. J. Biol. Chem. 277, 41906–41915 (2003).
Newhouse, B. J. Tryptase inhibitiors — Review of the recent patent literature. IDrugs 5, 682–688 (2002).
Boye, J. A. Mast cells: beyond IgE. J. Allergy Clin. Immunol. 111, 24–32 (2003).
Duan, W. et al. Antiinflammatory effects of genistein, a tyrosine kinase inhibitor on a guinea pig model of asthma. Am. J. Respir. Crit. Care Med. 167, 185–192 (2003).
Katz, H. R. et al. Mouse mast cell gp 49B1 contains two immunoreceptor tyrosine-based inhibition motifs and suppresses mast cell activation when colligated with the high affinity receptor for IgE. Proc. Natl Acad. Sci. USA 93, 10809–10814 (1996). Receptors linked to immuno-receptor typosine-based inhibitory motifs such as gp49B can be harnessed to disable mast cell activation–secretion coupling.
Daheshia, M. et al. Increased severity of local and systemic anaphylactic reactions in gp49B1-deficient mice. J. Exp. Med. 194, 227–233 (2001).
Costa, J. J. et al. Recombinant human stem cell factor (kit ligand) promotes human mast cells and melanocyte hyperplasia and functional activation in vivo. J. Exp. Med. 183, 2681–2686 (1996).
Tatton, l., Morley, G. M., Chopra, R. & Khwaja, A. The Src-selective kinase inhibitor PP1 also inhibits Kit and B tyrosine kinases. J. Biol. Chem. 278, 4847–4853 (2003).
Duffy, S. M., Lawley, W. J., Conley, E. C. & Bradding, P. Resting and activation-dependent ion channels in human mast cells. J. Immunol. 167, 4261–4270 (2001). The identification of specific ion channels on mast cells linked to cell activation offers a new way to stabilize mast cells with selective blockers.
Bradding, P., Okayama, Y., Kambe, N. & Saito, H. Ion channel gene expression in human lung, skin and cord blood derived mast cells. J. Leukoc. Biol. 73, 614–620 (2003).
Baraniak, S. N. Sensory parasympathetic and sympathetic neural influences in the nasal mucosa. J. Allergy Clin. Immunol. 90, 1045–1050 (1992).
Barnes, P. J. Neurogenetic inflammation in the airways. Respir. Physiol. 125, 145–154 (2001).
Fajac, I., Braunstein, G., Ickovic, M. R., Lacranicque, J. & Frossard, N. Selective recruitment of eosinophils by substance P after repeated allergen exposure in allergic rhinitis. Allergy 50, 970–975 (1995).
Austen, C. E., Foreman, J. C. & Scadding, G. H. Reduction by Hoe 140, the β2 kinin receptor antagonist, of antigen-induced nasal blockage. Br. J. Pharmacol. 111, 969–971 (1994).
Uddman, R., Cantera, L., Cardell, L. O. & Edvinnsson, L. Expression of NPY Y1 and CGRP1 receptors in human nasal mucosa: implications in allergic rhinitis. Ann. Otol. Rhinol. Laryngol. 108, 969–973 (1999).
Korsegren, M. et al. Neural expression and increased lavage fluid levels of secretoneurin in seasonal allergic rhinitis. Am. J. Respir. Crit. Care Med. 167, 1504–1508 (2003).
Duzendorfer, S. et al. Secretoneurin, a novel neuropeptide is a potent chemoattractant for human eosinophils. Blood 91, 1527–1532 (1998). The importance of neurogenic inflammation in chronic rhinitis is highlighted by the discovery of secretoneurin that has potent eosinophil chemo-attractant properties.
Frew, A. J. Immunotherapy of allergy disease. J. Allergy Clin. Immunol. 111, S712–S719 (2003).
Nelson, H. S. Advances in upper airway diseases and allergen immunotherapy. J. Allergy Clin. Immunol. 111, S793–S798 (2003).
Moller, C. et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT study). J. Allergy Clin. Immunol. 109, 251–256 (2002).
Pajno, G. B., Barberio, G., De Luca, F., Morabito, L. & Parmiani, S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clin. Exp. Allergy 31, 1392–1397 (2001).
Malling, H. J. Is sublingual immunotherapy clinically effective? Curr. Opin. Allergy Clin. Immunol. 2, 523–531 (2002).
Durham, S. R. et al. Long-term clinical efficacy of grass-pollen immunotherapy. N. Engl. J. Med. 341, 468–475 (1999). This key study illustrates the long-term benefits obtained with allergen-specific immunotherapy in allergic rhinitis once therapy has stopped, with remission extending to greater than three years.
Di Rienzo, V. et al. Long-lasting effect of sub-lingual immunotherapy in children with asthma due to house dust mite: a 10 year prospective study. Clin. Exp. Allergy 33, 206–210 (2003).
Canonica, G. W. & Passalacqua, G. Non-injection routes for immunotherapy. J. Allergy Clin. Immunol. 111, 437–448 (2003).
Walker, S. M., Verhoef, A., Till, S. J. & Durham, S. R. Grass pollen immunotherapy for hayfever is associated with increases in local nasal but not peripheral TH1:TH2 cyotkine ratios. Immunology 105, 56–62 (2002).
Nasser, S. M., Ying, S., Meng, Q., Kay, A. B. & Ewan, P. W. Interleukin-10 levels increase in cutaneous biopsies of patients undergoing wasp venom immunotherapy. Eur. J. Immunol. 31, 3704–3713 (2001).
McHugh, R. S. & Shevach, E. M. The role of suppressor T cells in regulation of immune responses. J. Allergy Clin. Immunol. 110, 693–702 (2002).
TePas, E. C. & Umetsu, D. T. Immunotherapy of asthma and allergic disease. Curr. Opin. Pediatr. 12, 574–578 (2000).
Hamid, Q. A., Schotman, E., Jacobsen, M. R., Walker, S. M. & Durham, S. R. Increases in IL-12 messenger RNA+ cells accompany inhibition of allergen-induced late skin responses after successful grass pollen immunotherapy. J. Allergy Clin. Immunol. 99, 254–269 (1997).
Yazdanbahsh, M., Kremsner, P. G. & van Ree, R. Allergy parasites and the hygiene hypothesis. Science 296, 490–494 (2002).
Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. Fox P3 programs the development and function of CD4+CD25+ regulatory T cells. Nature Immunol. 4, 330–336 (2003).
Shimizu, J. et al. Stimulation of CD25+ CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nature Immunol. 3, 135–142 (2002).
Bystry, R. S., Aluvihare, U., Welch, K. A., Kallikoardis, M. & Betz, A. G. B cells and professional APCs recruit regulatory T cells via CCL4. Nature Immunol. 2, 1126–1132 (2001).
Ebner, C. Immunological mechanisms operative in allergen-specific immunotherapy. Int. Arch. Allergy Immunol. 119, 1–5 (1999). This review summarizes some of the immunological pathways that are harnessed in the beneficial effects of allergen immunotherapy.
Briner, T. J., Kuo, M. C., Keating, K. M., Rogers, B. L. & Greenstein, J. L. Peripheral T-cell tolerance induced in naive and primed mice by subcutaneous injection of peptides form the major cat allergen Fel d 1. Proc. Natl Acad. Sci. USA 90, 7608–7612 (1993).
Norman, P. S. et al. Treatment of cat allergy with T cell reactive peptides. Am. J. Resp. Crit. Care Med. 154, 1623–1628 (1996).
Oldfield, W. L., Larché, M. & Kay, A. B. Effect of T-cell peptides derived from fel D 1 on allergic reactions and cytokine production in patients sensitive to cats: a randomised controlled trial. Lancet 360, 47–53 (2002).
Haselden, B. M., Kay, A. B. & Larché, M. IgE-dependent MHC-restricted T cell peptide epitope-induced late asthmatic reactions. J. Exp. Med. 189, 1885–1894 (1999).
Haselden, B. M. et al. Late asthmatic reactions provoked by intradermal injection of T-cell peptide epitopes are not associated with bronchial mucosal infiltration of eosinophils or TH2-type cells or with elevated concentrations of histamine or eicosanoids in bronchoalveolar fluid. J. Allergy Clin. Immunol. 108, 394–401 (2001).
Broide, D. H. & Raz, E. in Lung Biology in Health and Disease Vol 177 (eds Eissa, N. T. & Huston, D. P.) 839–865 (Marcel Dekker, New York, in the press).
Horner, A. A., Van Uden, J. H., Zubeldia, J. M., Broide, D. & Raz, E. DNA-based immunotherapeutics for the treatment of allergic disease. Immunol. Rev. 179, 102–118 (2001). This review summarizes the interactions between non-methylated CpG oligonucleotides from microorganisms and protective pathways in allergic disease through an interaction with TLR9.
Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000).
Roman, M. et al. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nature Med. 3, 849–854 (1997).
Horner, A. A. et al. Immmunostimulatory DNA inhibits IL-4 dependent IgE synthesis by human B cells. J. Allergy Clin. Immunol. 108, 417–423 (2001).
Broide, D. et al. Immunostimulatory DNA sequences inhibit IL-5, eosinophilic inflammation and airway hyperresponsiveness in mice. J. Immunol. 161, 7054–7062 (1998).
Kline, J. N. et al. Modulation of airway inflammation by CpG oligodeoxynucleotides in a murine model of asthma. J. Immunol. 160, 2555–2559 (1998).
Ikeda, R. K. et al. Resolution of airway inflammation following ovalbumin inhalation: comparison of ISS DNA and corticosteroids. Am. J. Resp. Cell Mol. Biol. 28, 655–683 (2003).
Hussain, I. et al. Modulation of murine allergic rhinosinusitis by CpG oligodeoxynucleotides. Layrngoscope 112, 1819–1826 (2002).
Creticos, P. et al. Immunotherapy with immunostimulatory oligonucleotides linked to purified ragweed Amb a 1 allergen: Effects on antibody production, nasal allergen provocation, and ragweed seasonal rhinitis. J. Allergy Clin. Immunol. 109, 743–744 (2002). Immunotherapy with covalently linked CpG oligonucleotides with the major ragweed allergen has improved efficacy compared with allergen alone.
Henderson, W. R. Jr, Chi, E. Y. & Maliszewski, C. R. IL-4 receptor inhibits airway inflammation following allergen challenge in a mouse model of asthma. J. Immunol. 164, 1086–1095 (2000).
Cieslewicz, G. et al. The late, but not early, asthmatic response is dependent on IL-5 and correlates with eosinophil infiltration. J. Clin. Invest. 104, 301–308 (1999).
Marshall, J. D. et al. Immunostimulatory sequence DNA linked to the Amb a 1 allergen promotes TH1 cytokine expression while downregulating TH2 cytokine expression in PBMCs from human patients with ragweed allergy. J. Allergy Clin. Immunol. 108, 191–197 (2001).
Tighe, H. et al. Conjugation of immunostimulatory DNA to the short ragweed allergen Amb a 1 enhances it immunogenicity and reduces its allergenicity. J. Allergy Clin. Immunol. 106, 124–134 (2000).
Chang, T. W. The pharmacological basis of anti-IgE therapy. Nature Biotechnol. 18, 157–163 (2000).
Presta, S. R. et al. Humanization of an antibody directed against IgE. J. Immunol. 151, 2623–2632 (1993).
Lazaar, A. L. Technology evaluation omalizumab. Genetech/ Novartis/Tanox. Curr. Opin. Mol. Ther. 5, 81–89 (2003).
Casale, T. B. et al. Effect of omalizumab on symptoms of seasonal allergic rhinitis: a randomized controlled trial. JAMA 286, 2956–2967 (2001). A new approach to treating allergic rhinitis and associated atopic disease is described using a monoclonal antibody directed to the Fc portion of IgE to prevent its binding to receptors on mast cells.
Adelroth, E. et al. Recombinant humanized on A6-E25, an anti-IgE mAb, in birch-pollen-induced seasonal allergic rhinitis. J. Allergy Clin. Immunol. 106, 253–259 (2000).
Kuehr, J. et al. Efficacy of combination treatment with anti-IgE plus specific immunotherapy in polysensitized children and adolescents with seasonal allergic rhinitis. J. Allergy Clin. Immunol. 109, 274–280 (2002). A combination of anti-IgE and allergen-specific immunotherapy (SIT) improves efficacy and offers promise for the use of IgE blockade to increase protection against the side effects of SIT.
Plewako, H. et al. The effect of omalizumab on nasal allergic inflammation. J. Allergy Clin. Immunol. 110, 68–71 (2002).
MacGlashan, D. W. Jr. et al. Down regulation of FcεR1 expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J. Immunol. 158, 1438–1445 (1997).
Novak, N., Kraft, S. & Bieber, T. Unravelling the mission of FcεR1 on antigen presenting cells. J. Allergy Clin. Immunol. 111, 38–44 (2003).
Vernesson, M., Ledin, A., Johansson, J. & Hellman, L. Generation of therapeutic antibody response against IgE through vaccination. FASEB J. 16, 875–877 (2002).
Nadler, M. J. & Kinet, J. P. Uncovering new complexities in mast cell signalling. Nature Immunol. 3, 707–708 (2002).
Christodoulopulos, P., Cameron, L., Durham, S. & Hamid, Q. Molecular pathology of allergic disease. II: Upper airway disease. J. Allergy Clin. Immunol. 105, 211–223 (2000).
Wills-Karp, M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 17, 255–281 (1999).
Foster, P. S. et al. Interleukin-5 and eosinophils as therapeutic targets for asthma. Trends Mol. Med. 8, 162–167 (2002).
Borish, L. C et al. Efficacy of soluble IL-4 receptor for the treatment if adults with asthma. J. Allergy Clin. Immunol. 107, 963–970 (2001). An understanding of the Fc ε ;R1- and Fc ε R2-binding sites on human IgE has created a new opportunity for the development of a peptide vaccine for treating atopic diseases.
Borrish, L. C. et al. Interleukin-4 receptor in moderate atopic asthma. A phase 1/randomized, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 160, 1816–1823 (1999).
Atamas, S. P., Choi, J., Yurovsky, V. V. & White, B. An alternative splice variant of human IL-4, IL-4 inhibits IL-4 stimulated T cell proliferation. J. Immunol. 156, 435–441 (1996).
Kruse, N., Tony, H. P. & Sebald, W. Conversion of human IL-4 into a high affinity antagonist by a single aminoacid replacement. EMBO J. 11, 3237–3244 (1992).
Wills-Karp, M. et al. Interleukin 13: central mediator of allergic asthma. Science 282, 2258–2261 (1998).
Skowron, M., Peret, E., Marano, F., Caput, D. & Tournier, F. Intelruekin-13 alters mucociliary differentiation of human nasal epithelial cells. Chest 123, 373S–374S (2003).
Wynn, T. A. IL-13 effector functions. Annu. Rev. Immunol. 21, 425–456 (2003). This review summarizes the key features of IL-13 as a major therapeutic target in chronic allergic diseases that include immunological, inflammatory and structural cell effects.
Kleinjan, A. et al. Increase in IL-8, IL-10, IL-13 and RANTES mRNA levels (in situ hybridization) in the nasal mucosa after nasal allergen provocation. J. Allergy Clin. Immunol. 103, 441–450 (1999).
Andrews, A. L., Holloway, J. W., Puddicombe, S. L., Holgate, S. T. & Davies, D. E. Kinetic analysis of interleukin-13 receptor complex. J. Biol. Chem. 277, 46073–46078 (2002).
Wood, N. et al. Enhanced interleukin (IL)-13 responses in mice lacking IL-13 receptor α2. J. Exp. Med. 197, 703–709 (2003).
Wills-Karp, M. & Chiaramonte, M. Interleukin-13 in asthma. Curr. Opin. Pulm. Med. 9, 21–27 (2003).
Wills-Karp, M. Murine models of asthma in understanding immune dysregulation in human asthma. Immopharmacology 48, 263–268 (2000).
Oshima, Y. & Puri, R. A novel antagonist that blocks the biological activity of human IL-13 in immune and non-immune cells. FASEB J. 15, 1469–1471 (2001).
Leckie, M. J. et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyperresponsiveness and the late asthmatic response. Lancet 356, 2144–2148 (2000). This report describes the failure of IL-5 blockade to attenuate allergen-provoked late-phase allergic responses in the airways despite depleting circulating and tissue eosinophils.
Kips, J. C. et al. Effect of SECH 55700, a humanized anti-human interleukin-5 antibody in severe persistent asthma. Am. J. Respir. Crit. Care Med. 167, 1655–1659 (2003).
Flood-Page, P. T., Menzies-Gow, A. N., Kay, A. B. & Robinson, D. S. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am. J. Respir. Crit. Care Med. 167, 199–204 (2003).
Saito, H. et al. Pathogenesis of murine experimental allergic rhinitis: a study of local and systemic consequences of IL-5 deficiency. J. Immunol. 168, 3017–3023 (2003).
Zhou, C. Y., Crocker, I. C., Koenig, G., Romero, F. A. & Townley, R. G. Anti-interleukin-4 inhibits IgE production in a murine model of atopic asthma. J. Asthma 34, 195–201 (1997).
Tanaka, H., Nagai, H. & Macda, Y. Effect of anti IL-4 and anti-IL-5 antibodies on allergic airway hyperresponsiveness in mice. Life Sci. 62, PL169–PL174 (1998).
Terada, N. et al. The kinetics of allergen-induced eotaxin level in nasal lavage fluid: its key role in eosinophil recruitment in nasal mucosa. Am. J. Respir. Crit. Care Med. 164, 575–579 (2001). Eotaxins are chemokines with properties linked to eosinophil recruitment into the nasal mucosa in rhinitis.
Gorski, P. et al. Eotaxin but not MCP-3 induces eosinophil influx into nasal fluid in allergic patients. Allergy 57, 519–528 (2002).
Cooper, J. A. Jr., Ridgeway, A. L., Pearson, J. & Culbreth, R. R. Attenuation of interleukin 8-induced nasal inflammation by an inhibitor peptide. Am. J. Respir. Crit. Care Med. 163, 1198–1205 (2001).
Mattes, J. et al. Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreacitivity in experimental asthma. J. Exp. Med. 195, 1433–1444 (2002).
Von Hertzen, L. & Haahtela, T. Could the risk of asthma and atopy be reduced by a vaccine that induces a strong T-helper Type 1 response? Am. J. Respir. Cell Mol. Biol. 22, 139–142 (2000).
Barnes, P. J. Cytokine-directed therapies in asthma. Allergol. Int. 52, 53–63 (2003).
Bryan, S. A. et al. Effects of recombinant human interleukin-12 on eosinophils, airway hyperresponsiveness and the late asthmatic response. Lancet 356, 2149–2153 (2000).
Castro, M., Chaplin, D. D., Walter, M. J. & Holtzman, M. J. Could asthma be worsened by stimulation of the T-helper Type 1 immune response? Am. J. Respir. Cell Mol. Biol. 22, 143–146 (2000). Although the hygiene hypothesis has suggested augmentation of T H 1 responses as a possible mechanism for the prevention of disease, this publication draws attention to the possible downside of this approach in established allergic disease.
Zuang-Amorim, C. et al. Long-term protective and antigen-specific effect of heat killed Mycobacterium vaccae in a murine model of allergic pulmonary inflammation. J. Immunol. 169, 1492–1499 (2002).
Camporota, L. et al. The effect of Mycobacterium vaccae on allergen-induced airway response in atopic asthma. Eur. Respir J. 21, 287–293 (2003).
Zuang-Amorin, C. et al. Suppression of airway eosinophila by killed mycobacterium vaccae-induced allergen-specific regulatory T cells. Nature Med. 8, 625–629 (2002).
Kalliomäki, M. et al. Probiotics in the primary prevention of atopic disease: a randomised placebo controlled trial. Lancet 357, 1076–1079 (2001).
Helin, T., Haahtela, S. & Haahtela, T. No effect of oral treatment with an intestinal bacterial strain, Lactobacillus rhamnosus (ATCC 53103) on birch pollen allergy: a placebo-controlled double-blind study. Allergy 57, 243–246 (2002).
Braunstahl, G. H. et al. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airways. J. Allergy Clin. Immunol. 107, 469–476 (2001).
Symon, F. A., Walsh, G. M., Watson, S. R. & Wardlaw, A. J. Eosinophil adhesion to nasal polyp endothelium is P-selectin dependent. J. Exp. Med. 180, 371–376 (1994).
Broide, D. H., Sullivan, S., Gifford, T. & Sriraarao, P. Inhibition of pulmonary eosinophilia in P-selectin- and ICAM-1-deficient mice. Am. J. Respir. Cell Mol. Biol. 18, 218–225 (1998). Vascular adhesion molecules are good therapeutic targets in chronic allergic rhinitis. This paper describes the pivotal role of P-selection with ICAM–1 in the recruitment in the recruitment of leukocytes during the allergic cascade.
Sriramarao, P. et al. E-selectin preferentially supports neutrophils but not eosinophil rolling under conditions of flow in vitro and in vivo. J. Immunol. 157, 4672–4680 (1996).
Jackson, D. Y. α4 integrin antagonists. Curr. Pharm. Des. 8, 1229–1253 (2002).
Anwar, A. R., Moqbel, R., Walsh, G. M., Kay, A. B. & Wardlaw, A. J. Adhesion to fibronectin prolongs eosinophil survival. J. Exp. Med. 177, 839–843 (1993).
Henderson, W. R. Jr. et al. Blockade of CD49d (α4 integrin) on intrapulmonary but not circulating leukocytes inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. J. Clin. Invest. 100, 3083–3092 (1997).
Abraham, W. M. et al. A small-molecule, tight-binding inhibitor of the integrin α4β1 blocks antigen-induced airway responses and inflammation in experimental asthma in sheep. Am. J. Respir. Crit. Care Med. 162, 603–661 (2000).
Giembycz, C. A. Phosphodiesterase 4 inhibitors and the treatment of asthma. Drugs 59, 193–212 (2002).
Schmidt, B. M. et al. The phosphodiesterase 4 inhibitor rofulimast is effective in the treatment of allergic rhinitis. J. Allergy Clin. Immunol. 108, 503–506 (2001).
Sorbera, L. A., Leeson, P. A. & Castaner, J. Rofumilast, BY-217. Drugs of the Future 25, 1261–1264 (2000).
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
LocusLink
Glossary
- DESENSITIZE
-
The use of allergens or modified allergens to induce immunological tolerance.
- CHROMONES
-
Anti-allergic drugs originally derived from Rhellin from the plant Amni visnaga.
- ATOPY
-
The genetic susceptibility to produce IgE antibodies against common environmental allergens.
- CpG DNA
-
Also known as immunostimulatory sequences (ISS), these are sequence-specific non-methylated DNA molecules of cytosine and guanosine, modelled on those present in microorganisms, which have the capacity to stimulate Toll-like receptor-9 found on antigen-presenting cells and to modify immune responses.
- AUTACOID
-
Low-molecular-weight mediators that participate in cell–cell communication.
- EICOSANOID
-
Mediators derived from polysaturated fatty acids.
- LEUKOCYTE IMMUNOGLOBULIN-LIKE RECEPTORS
-
Cell-surface molecules whose activation leads to the phosphorylation of immuno-receptor tyrosine-based motifs (ITIMS) on adjacent receptors such as FcεR1.
- SIT
-
Allergen-specific immunotherapy that uses incremental small doses of subcutaneously injected allergen to induce immunological tolerance.
- SLIT
-
Sublingual immunotherapy, which uses a higher concentration of allergen than used in SIT to induce tolerance via the buccal mucosa and draining lymphoid tissue.
- AMB A 1
-
The major allergen of ragweed pollen.
- FCεR1 AND FCεR2
-
The high- and low-affinity receptors for IgE present on mast cells, basophils and dendritic cells.
- ISOTYPE SWITCHING
-
The capacity of B lymphocytes to redirect their immunoglobulin (Ig) synthesis from IgM to another Ig subclass
- CAMS
-
Cell adhesion molecules including intercellular (ICAM) and vascular (VCAM) members that are expressed on endothelial cells, upregulated by cytokines and involved in leukocyte adhesion and activation.
- MYCOBACTERIUM VACCAE
-
A non-disease-causing mycobacterium found in the soil as a saprophyte.
- SELECTINS
-
Glycoproteins that are expressed on endothelial cells involved in leukocyte rolling (P selectin) or adhesion (E-selectin).
- VERY LATE ANTIGEN-4
-
(VLA-4). An integrin α4β1 that selectively binds to VCAM-1 and to CS-1 region of fibronectin.
Rights and permissions
About this article
Cite this article
Holgate, S., Broide, D. New targets for allergic rhinitis — a disease of civilization. Nat Rev Drug Discov 2, 903–915 (2003). https://doi.org/10.1038/nrd1224
Issue Date:
DOI: https://doi.org/10.1038/nrd1224
This article is cited by
-
A 2-year step-down withdrawal from inhaled corticosteroids in asthmatic children receiving immunotherapy
World Journal of Pediatrics (2017)
-
Treatment of allergic rhinitis with acupoint herbal plaster: an oligonucleotide chip analysis
BMC Complementary and Alternative Medicine (2016)
-
Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma
Nature Reviews Immunology (2005)