Abstract
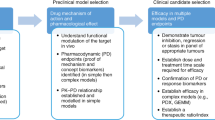
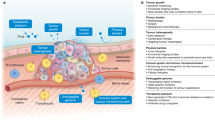
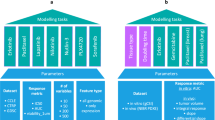
Oncology, as a therapeutic area, is characterized by a desperate medical need for new drugs; the use of drugs that kill cells and which are consequently often toxic; and rates of failure in expensive Phase III trials that eclipse many other disease areas. The poor performance of most investigational cancer drugs implies that the standard preclinical disease models are faulty or, at least, improperly used. Some studies, however, support the view that cancer models can be highly effective, but only when selected and interpreted with care.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kola, I. & Landis, J. Can the pharmaceutical industry reduce attrition rates? Nature Rev. Drug Discov. 3, 711–715 (2004).
Gorre, M. E. & Sawyers, C. L. Molecular mechanisms of resistance to STI571 in chronic myeloid leukemia. Curr. Opin. Hematol. 9, 303–307 (2002).
Kamb, A. Consequences of nonadaptive alterations in cancer. Mol. Biol. Cell. 14, 2201–2205 (2003).
Roberts Jr., T. G. et al. Trends in the risks and benefits to patients with cancer participating in phase 1 clinical trials. JAMA 292, 2130–2140 (2004).
Ishii, N., Robert, M., Nakayama, Y., Kanai, A. & Tomita, M. Toward large-scale modeling of the microbial cell for computer simulation. J. Biotechnol. 113, 281–294 (2004).
Oskoglou-Nomikos, T., Pater, J. L. & Seymour, L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin. Cancer Res. 9, 4227–4239 (2003).
Yingling, J. M., Blanchard, K. L. & Sawyer, J. S. Development of TGF-β signaling inhibitors for cancer therapy. Nature Rev. Drug Discov. 3, 1011–1022.
Peterson, J. K. & Houghton, P. J. Integrating pharmacology and in vivo cancer models in preclinical and clinical drug development. Eur. J. Cancer 40, 837–844 (2004).
Druker, B. J. Imatinib as a paradigm of targeted therapies. Adv. Cancer Res. 91, 1–30 (2004).
Dancey, J. E. Predictive factors for epidermal growth factor receptor inhibitors — the bull's-eye hits the arrow. Cancer Cell 5, 411–415 (2004).
Tuveson, D. A. et al. STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: biological and clinical implications. Oncogene 20, 5054–5058 (2001).
Griffin, J. D. FLT3 tyrosine kinase as a target in acute leukemias. Hematol. J. 5, S188–190 (2004).
Slamon, D. & Pegram, M. Rationale for trastuzumab (Herceptin) in adjuvant breast cancer trials. Semin. Oncol. 28, 13–19 (2001).
Weisberg, E. & Griffin, J. D. Resistance to imatinib (Glivec): update on clinical mechanisms. Drug Resist. Updat. 6, 231–238 (2003).
Scherf, U. et al. A gene expression database for the molecular pharmacology of cancer. Nature Genet. 24, 236–244 (2000).
Jonkers, J. & Berns, A. Conditional mouse models of sporadic cancer. Nature Rev. Cancer 2, 251–265.
Johnson, J. I. et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br. J. Cancer 84, 1424–1431 (2001)
Wong, S. L. et al. Proc. Natl Acad. Sci. USA 101, 15682–15687 (2004).
Author information
Authors and Affiliations
Related links
Related links
DATABASES
Entrez Gene
National Cancer Institute Cancer Topics
Rights and permissions
About this article
Cite this article
Kamb, A. What's wrong with our cancer models?. Nat Rev Drug Discov 4, 161–165 (2005). https://doi.org/10.1038/nrd1635
Issue Date:
DOI: https://doi.org/10.1038/nrd1635
This article is cited by
-
Novel organoid construction strategy for non-involuting congenital hemangioma for drug validation
Journal of Biological Engineering (2023)
-
Organoid models derived from patients with malignant phyllodes tumor of the breast
Breast Cancer Research and Treatment (2023)
-
The potential application of organoids in breast cancer research and treatment
Human Genetics (2022)
-
Automated microfluidic platform for dynamic and combinatorial drug screening of tumor organoids
Nature Communications (2020)
-
MiR-1976 knockdown promotes epithelial–mesenchymal transition and cancer stem cell properties inducing triple-negative breast cancer metastasis
Cell Death & Disease (2020)