Abstract
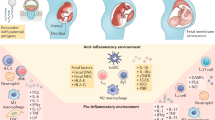
Pregnancy and the postpartum period have a profound effect on autoimmune thyroid disease. Graves disease ameliorates during pregnancy, only to relapse postpartum, whereas postpartum thyroiditis is caused by destructive thyroiditis during the first few months after delivery. The immunology of pregnancy underlies these changes: the mother must maintain tolerance of the fetal semi-allograft while not suppressing her own immune system and exposing herself and the fetus to infection. Nonspecific factors, including hormonal changes, trophoblast expression of key immunomodulatory molecules and a switch to a predominantly T-helper-2-type pattern of cytokines, play some part in the maintenance of transient tolerance to paternal antigens in pregnancy; however, the generation of specific regulatory T (TREG) cells is key to this maintenance. TREG cells preferentially accumulate in the decidua but may also be present in the mother's circulation and are thus capable of regulating coincidental autoimmune responses through the phenomenon of linked suppression. In turn, this suppression may explain why thyroid autoantibody levels decline during pregnancy, which leads to remission of Graves disease. Postpartum exacerbation of autoimmunity may reflect an imbalance in TREG cells, which is caused by the rapid fall in the numbers of these cells after delivery.
Key Points
-
Women are exposed to fetal alloantigens during pregnancy and must establish immunological tolerance to these antigens to prevent rejection of the fetus
-
A generalized reduction of maternal immune responsiveness occurs during pregnancy, which is caused by increased levels of progesterone
-
The trophoblast synthesizes a number of immunologically active molecules that suppress immune responses at the interface between mother and placenta
-
Most importantly, the mother generates regulatory T (TREG) cells early in pregnancy that maintain a state of tolerance to fetal alloantigens as long as the pregnancy continues
-
These TREG cells may ameliorate coincidental autoimmune thyroid diseases during pregnancy by linked suppression; worsening of these diseases postpartum may result from changes in the TREG cell and cytokine milieu
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Robertson, H. E. W. Lassitude, coldness and hair changes following pregnancy and their response to treatment with thyroid extract. Br. Med. J. 2 (Suppl.), S93–S98 (1948).
Cooke, R. T. Myxoedema in young women. J. Coll. Gen. Pract. 6, 626–630 (1963).
Amino, N., Miyai, K., Onishi, T., Hashimoto, T. & Arai, K. Transient hypothyroidism after delivery in autoimmune thyroiditis. J. Clin. Endocrinol. Metab. 42, 296–301 (1976).
Ginsberg, J. & Walfish P. G. Post-partum transient thyrotoxicosis with painless thyroiditis. Lancet 1, 1125–1128 (1977).
Chan, G. W. & Mandel, S. J. Therapy insight: management of Graves' disease during pregnancy. Nat. Clin. Pract. Endocrinol. Metab. 3, 470–478 (2007).
Prummel, M. F. & Wiersinga, W. M. Thyroid autoimmunity and miscarriage. Eur. J. Endocrinol. 150, 751–755 (2004).
Caturegli, P. et al. Autoimmune hypophysitis. Endocr. Rev. 26, 599–614 (2005).
Klein, L., Hinterberger, M., Wirnsberger, G. & Kyewski, B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat. Rev. Immunol. 9, 833–844 (2009).
Ulmanen, I., Halonen, M., Ilmarinen, T. & Peltonen, L. Monogenic autoimmune diseases—lessons of self-tolerance. Curr. Opin. Immunol. 17, 609–615 (2005).
Mueller, D. L. Mechanisms maintaining peripheral tolerance. Nat. Immunol. 11, 21–27 (2010).
Germain, R. N. Special regulatory T-cell review: a rose by any other name: from suppressor T cells to Tregs, approbation to unbridled enthusiasm. Immunology 123, 20–27 (2008).
Penhale, W. J., Irvine, W. J., Inglis, J. R. & Farmer, A. Thyroiditis in T cell-depleted rats: suppression of the autoallergic response by reconstitution with normal lymphoid cells. Clin. Exp. Immunol. 25, 6–16 (1976).
Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155, 1151–1164 (1995).
Shimizu, J., Yamazaki, S., Takahashi, T., Ishida, Y. & Sakaguchi, S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 3, 135–142 (2002).
Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003).
Morris, G. P., Brown, N. K. & Kong, Y. C. Naturally-existing CD4(+)CD25(+)Foxp3(+) regulatory T cells are required for tolerance to experimental autoimmune thyroiditis induced by either exogenous or endogenous autoantigen. J. Autoimmun. 33, 68–76 (2009).
Billington, W. D. The immunological problem of pregnancy: 50 years with the hope of progress. A tribute to Peter Medawar. J. Reprod. Immunol. 60, 1–11 (2003).
Adams Waldorf, K. M. & Nelson, J. L. Autoimmune disease during pregnancy and the microchimerism legacy of pregnancy. Immunol. Invest. 37, 631–644 (2008).
Guleria, I. & Sayegh, M. H. Maternal acceptance of the fetus: true human tolerance. J. Immunol. 178, 3345–3351 (2007).
Tafuri, A., Alferink, J., Möller, P., Hämmerling, G. J. & Arnold, B. T cell awareness of paternal alloantigens during pregnancy. Science 270, 630–633 (1995).
Arck, P., Hansen, P. J., Mulac Jericevic, B., Piccinni, M. P. & Szekeres-Bartho, J. Progesterone during pregnancy: endocrine-immune cross talk in mammalian species and the role of stress. Am. J. Reprod. Immunol. 58, 268–279 (2007).
Murphy, S. P., Choi, J. C. & Holtz, R. Regulation of major histocompatibility complex class II gene expression in trophoblast cells. Reprod. Biol. Endocrinol. 2, 52 (2004).
Rogers, A. M., Boime, I., Connolly, J., Cook, J. R. & Russell, J. H. Maternal-fetal tolerance is maintained despite transgene-driven trophoblast expression of MHC class I, and defects in Fas and its ligand. Eur. J. Immunol. 28, 3479–3487 (1998).
Erlebacher, A., Vencato, D., Price, K. A., Zhang, D. & Glimcher, L. H. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J. Clin. Invest. 117, 1399–1411 (2007).
Hunt, J. S., Langat, D. K., McIntire, R. H. & Morales, P. J. The role of HLA-G in human pregnancy. Reprod. Biol. Endocrinol. 4 (Suppl. 1), S10 (2006).
Le Bouteiller, P. & Piccinni, M. P. Human NK cells in pregnant uterus: why there? Am. J. Reprod. Immunol. 59, 401–406 (2008).
Munn, D. H. et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281, 1191–1193 (1998).
Baban, B. et al. Indoleamine 2,3-dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J. Reprod. Immunol. 61, 67–77 (2004).
Paiva, P., Menkhorst, E., Salamonsen, L. & Dimitriadis, E. Leukemia inhibitory factor and interleukin-11: critical regulators in the establishment of pregnancy. Cytokine Growth Factor Rev. 20, 319–328 (2009).
Raghupathy, R. Th1-type immunity is incompatible with successful pregnancy. Immunol. Today 18, 478–482 (1997).
White, C. A., Johansson, M., Roberts, C. T., Ramsay, A. J. & Robertson, S. A. Effect of interleukin-10 null mutation on maternal immune response and reproductive outcome in mice. Biol. Reprod. 70, 123–131 (2004).
Fitzgerald, J. S., Toth, B., Jeschke, U., Schleussner, E. & Markert, U. R. Knocking off the suppressors of cytokine signaling (SOCS): their roles in mammalian pregnancy. J. Reprod. Immunol. 83, 117–123 (2009).
Aluvihare, V. R., Kallikourdis, M. & Betz, A. G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 5, 266–271 (2004).
Zenclussen, A. C. et al. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am. J. Pathol. 166, 811–822 (2005).
Sasaki, Y. et al. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol. Hum. Reprod. 10, 347–353 (2004).
Somerset, D. A., Zheng, Y., Kilby, M. D., Sansom, D. M. & Drayson, M. T. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology 112, 38–43 (2004).
Heikkinen, J., Möttönen, M., Alanen, A. & Lassila, O. Phenotypic characterization of regulatory T cells in the human decidua. Clin. Exp. Immunol. 136, 373–378 (2004).
Tilburgs, T. et al. Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J. Immunol. 180, 5737–5745 (2008).
Schumacher, A. et al. Human chorionic gonadotropin attracts regulatory T cells into the fetal-maternal interface during early human pregnancy. J. Immunol. 182, 5488–5497 (2009).
Shao, L., Jacobs, A. R., Johnson, V. V. & Mayer, L. Activation of CD8+ regulatory T cells by human placental trophoblasts. J. Immunol. 174, 7539–7547 (2005).
Polanczyk, M. J., Hopke, C., Huan, J., Vandenbark, A. A. & Offner, H. Enhanced FoxP3 expression and Treg cell function in pregnant and estrogen-treated mice. J. Neuroimmunol. 170, 85–92 (2005).
Mjösberg, J., Berg, G., Jenmalm, M. C. & Ernerudh, J. FOXP3+ regulatory T cells and T helper 1, T helper 2, and T helper 17 cells in human early pregnancy decidua. Biol. Reprod. 82, 698–705 (2010).
Mellor, A. L. & Munn, D. H. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 4, 762–774 (2004).
Mjösberg, J. et al. Systemic reduction of functionally suppressive CD4dimCD25highFoxp3+ Tregs in human second trimester pregnancy is induced by progesterone and 17beta-estradiol. J. Immunol. 183, 759–769 (2009).
Wafula, P. O. et al. PD-1 but not CTLA-4 blockage abrogates the protective effect of regulatory T cells in a pregnancy murine model. Am. J. Reprod. Immunol. 62, 283–292 (2009).
Santner-Nanan, B. et al. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J. Immunol. 183, 7023–7030 (2009).
Mold, J. E. et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 322, 1562–1565 (2008).
Imaizumi, M. et al. Pregnancy and murine thyroiditis: thyroglobulin immunization leads to fetal loss in specific allogeneic pregnancies. Endocrinology 142, 823–829 (2001).
Lee, Y. L. et al. Increased fetal abortion rate in autoimmune thyroid disease is related to circulating TPO autoantibodies in an autoimmune thyroiditis animal model. Fertil. Steril. 91 (Suppl. 5), 2104–2109 (2009).
Imaizumi, M. et al. Non-MHC driven exacerbation of experimental thyroiditis in the postpartum period. Autoimmunity 34, 95–105 (2001).
McClain, M. A. et al. Pregnancy suppresses experimental autoimmune encephalomyelitis through immunoregulatory cytokine production. J. Immunol. 179, 8146–8152 (2007).
Wang, C. et al. Oestrogen modulates experimental autoimmune encephalomyelitis and interleukin-17 production via programmed death 1. Immunology 126, 329–335 (2009).
Shi, X. et al. Circulating lymphocyte subsets and regulatory T cells in patients with postpartum thyroiditis during the first postpartum year. Clin. Exp. Med. 9, 263–267 (2009).
Al-Shammri, S. et al. Th1/Th2 cytokine patterns and clinical profiles during and after pregnancy in women with multiple sclerosis. J. Neurol. Sci. 222, 21–27 (2004).
Kokandi, A. A., Parkes, A. B., Premawardhana, L. D., John, R. & Lazarus, J. H. Association of postpartum thyroid dysfunction with antepartum hormonal and immunological changes. J. Clin. Endocrinol. Metab. 88, 1126–1132 (2003).
Weetman, A. P. Immune reconstitution syndrome and the thyroid. Best Pract. Res. Clin. Endocrinol. Metab. 23, 693–702 (2009).
Carp, H. J., Meroni, P. L. & Shoenfeld, Y. Autoantibodies as predictors of pregnancy complications. Rheumatology (Oxford) 47 (Suppl. 3), 6–8 (2008).
Vukusic, S. et al. The Prevention of Post-Partum Relapses with Progestin and Estradiol in Multiple Sclerosis (POPART'MUS) trial: rationale, objectives and state of advancement. J. Neurol. Sci. 286, 114–118 (2009).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Weetman, A. Immunity, thyroid function and pregnancy: molecular mechanisms. Nat Rev Endocrinol 6, 311–318 (2010). https://doi.org/10.1038/nrendo.2010.46
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2010.46
This article is cited by
-
Maternal preconception thyroid autoimmunity is associated with neonatal birth weight conceived by PCOS women undergoing their first in vitro fertilization/intracytoplasmic sperm injection
Journal of Ovarian Research (2023)
-
Which endometrial preparation protocol provides better pregnancy and perinatal outcomes for endometriosis patients in frozen-thawed embryo transfer cycles? A retrospective study on 1413 patients
Journal of Ovarian Research (2023)
-
Thyroid autoimmunity following alemtuzumab treatment in multiple sclerosis patients: a prospective study
Clinical and Experimental Medicine (2023)
-
Modulating gut microbiota in a mouse model of Graves’ orbitopathy and its impact on induced disease
Microbiome (2021)
-
The COVID-19 Pandemic: an Appraisal of its Impact on Human Immunodeficiency Virus Infection and Pre-Eclampsia
Current Hypertension Reports (2021)