Key Points
-
When assessing neuroendocrine tumours (NETs), which comprise a wide variety of tumours with different imaging characteristics, morphological imaging (mostly CT or MRI) and nuclear medicine techniques are complementary
-
CT and MRI are typically used to evaluate a patient's response to therapy; however, a role for nuclear medicine techniques, aside from establishing progressive disease by visualizing new lesions, has yet to be defined
-
Somatostatin receptor imaging with radiolabelled somatostatin analogues is mandatory to determine whether patients are eligible for peptide receptor radionuclide therapy
-
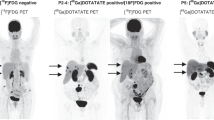
111In-pentetreotide scintigraphy is currently the most widely used method to assess somatostatin receptor expression, but 68Ga-DOTA-somatostatin analogue PET–CT could become the nuclear medicine test of choice for staging of patients with well-differentiated NETs
-
PET–CT with 18F-dihydroxy-L-phenylalanine and 11C-hydroxy-L-tryptophan might potentially be used in the future for therapy response evaluation
-
18F-FDG-PET is only recommended in patients with grade 3 neuroendocrine cancers but shows potential for other indications, for example, to predict prognosis and determine treatment schedule
Abstract
In patients with neuroendocrine tumours (NETs), a combination of morphological imaging and nuclear medicine techniques is mandatory for primary tumour visualization, staging and evaluation of somatostatin receptor status. CT and MRI are well-suited for discerning small lesions that might escape detection by single photon emission tomography (SPECT) or PET, as well as for assessing the local invasiveness of the tumour or the response to therapy. Somatostatin receptor imaging, by 111In-pentetreotide scintigraphy or PET with 68Ga-labelled somatostatin analogues, frequently identifies additional lesions that are not visible on CT or MRI scans. Currently, somatostatin receptor scintigraphy with 111In-pentetreotide is the more frequently available of the two techniques to determine somatostatin receptor expression and is needed to select patients for peptide receptor radionuclide therapy. In the future, because of its higher sensitivity, PET with 68Ga-labelled somatostatin analogues is expected to replace somatostatin receptor scintigraphy. Whereas 18F-FDG-PET is only used in high-grade neuroendocrine cancers, PET–CT with 18F-dihydroxy-L-phenylalanine or 11C-5-hydroxy-L-tryptophan is a useful problem-solving tool and could be considered for the evaluation of therapy response in the future. This article reviews the role of imaging for the diagnosis and management of intestinal and pancreatic NETs. Response evaluation and controversies in NET imaging will also be discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Rindi, G. et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 449, 395–401 (2006).
Rindi, G. et al. TNM staging of midgut and hindgut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 451, 757–762 (2007).
Hamilton, S. R. & Aaltonen, L. A. (eds) World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System (IARC Press, Lyon, 2000).
Bilimoria, K. Y. et al. Clinicopathologic features and treatment trends of pancreatic neuroendocrine tumors: analysis of 9,821 patients. J. Gastrointest. Surg. 11, 1460–1467 (2007).
Bergsma, H. et al. Peptide receptor radionuclide therapy (PRRT) for GEP-NETs. Best Pract. Res. Clin. Gastroenterol. 26, 867–881 (2012).
Sundin, A., Garske, U. & Orlefors, H. Nuclear imaging of neuroendocrine tumours. Best Pract. Res. Clin. Endocrinol. Metab. 21, 69–85 (2007).
Sundin, A., Vullierme, M. P., Kaltsas, G. & Plöckinger, U. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: radiological examinations. Neuroendocrinology 90, 167–183 (2009).
Sundin, A. Radiological and nuclear medicine imaging of gastroenteropancreatic neuroendocrine tumours. Best Pract. Res. Clin. Gastroenterol. 26, 803–818 (2012).
Masselli, G. & Gualdi, G. CT and MR enterography in evaluating small bowel diseases: when to use which modality? Abdom. Imaging 38, 249–259 (2013).
Bailey, A. A. et al. Diagnosis and outcome of small bowel tumors found by capsule endoscopy: a three-center Australian experience. Am. J. Gastroenterol. 101, 2237–2243 (2006).
Gallotti, A. et al. Incidental neuroendocrine tumors of the pancreas: MDCT findings and features of malignancy. AJR Am. J. Roentgenol. 200, 355–362 (2013).
Poultsides, G. A. et al. Pancreatic neuroendocrine tumors: radiographic calcifications correlate with grade and metastasis. Ann. Surg. Oncol. 19, 2295–2303 (2012).
Ishikawa, T. et al. Usefulness of EUS combined with contrast-enhancement in the differential diagnosis of malignant versus benign and preoperative localization of pancreatic endocrine tumors. Gastrointest. Endosc. 71, 951–959 (2010).
Khashab, M. A. et al. EUS is still superior to multidetector computerized tomography for detection of pancreatic neuroendocrine tumors. Gastrointest. Endosc. 73, 691–696 (2011).
Versari, A. et al. Ga-68 DOTA-TOC PET, endoscopic ultrasonography, and multidetector CT in the diagnosis of duodenopancreatic neuroendocrine tumors: a single-centre retrospective study. Clin. Nucl. Med. 35, 321–328 (2010).
Atiq, M. et al. EUS-FNA for pancreatic neuroendocrine tumors: a tertiary cancer center experience. Dig. Dis. Sci. 57, 791–800 (2012).
Pais, S. A. et al. EUS for pancreatic neuroendocrine tumors: a single-center, 11-year experience. Gastrointest. Endosc. 71, 1185–1193 (2010).
Stark, D. D., Moss, A. A., Goldberg, H. I. & Deveney, C. W. CT of pancreatic islet cell tumors. Radiology 150, 491–494 (1984).
Rossi, P. et al. CT of functioning tumors of the pancreas. AJR Am. J. Roentgenol. 144, 57–60 (1985).
Van Hoe, L., Gryspeerdt, S., Marchal, G., Baert, A. L. & Mertens, L. Helical CT for the preoperative localization of islet cell tumors of the pancreas: value of arterial and parenchymal phase images. AJR Am. J. Roentgenol. 165, 1437–1439 (1995).
Procacci, C. et al. Nonfunctioning endocrine tumors of the pancreas: possibilities of spiral CT characterization. Eur. Radiol. 11, 1175–1183 (2001).
Fidler, J. L. et al. Preoperative detection of pancreatic insulinomas on multiphasic helical CT. AJR Am. J. Roentgenol. 181, 775–780 (2003).
Chiti, A. et al. Comparison of somatostatin receptor imaging, computed tomography and ultrasound in the clinical management of neuroendocrine gastro-entero-pancreatic tumours. Eur. J. Nucl. Med. 25, 1396–1403 (1998).
Kumbasar, B. et al. Imaging of neuroendocrine tumors: accuracy of helical CT versus SRS. Abdom. Imaging 29, 696–702 (2004).
Hubalewska-Dydejczyk, A. et al. 99mTc-EDDA/HYNIC-octreotate scintigraphy, an efficient method for the detection and staging of carcinoid tumours: results of 3 years' experience. Eur. J. Nucl. Med. Mol. Imaging 33, 1123–1133 (2006).
Cwikła, J. B. et al. Diagnostic imaging of carcinoid metastases to the abdomen and pelvis. Med. Sci. Monit. 10 (Suppl. 3), 9–16 (2004).
Thoeni, R. F., Mueller-Lisse, U. G., Chan, R., Do, N. K. & Shyn, P. B. Detection of small, functional islet cell tumors in the pancreas: selection of MR imaging sequences for optimal sensitivity. Radiology 214, 483–490 (2000).
Semelka, R. C., Custodio, C. M., Cem Balci, N. & Woosley, J. T. Neuroendocrine tumors of the pancreas: spectrum of appearances on MRI. J. Magn. Reson. Imaging 11, 141–148 (2000).
Shi, W. et al. Localization of neuroendocrine tumours with [111In]-DTPA-octreotide scintigraphy (Octreoscan): a comparative study with CT and MR imaging. QJM 91, 295–301 (1998).
Carlson, B., Johnson, C. D., Stephens, D. H., Ward, E. M. & Kvols, L. K. MRI of pancreatic islet cell carcinoma. J. Comput. Assist. Tomogr. 17, 735–740 (1993).
Dromain, C. et al. Detection of liver metastases from endocrine tumors: a prospective comparison of somatostatin receptor scintigraphy, computed tomography, and magnetic resonance imaging. J. Clin. Oncol. 23, 70–78 (2005).
Elias, D. et al. Hepatic metastases from neuroendocrine tumors with a “thin slice” pathological examination: they are many more than you think. Ann. Surg. 251, 307–310 (2010).
Rahmim, A. & Zaidi, H. PET versus SPECT: strengths, limitations and challenges. Nucl. Med. Commun. 29, 193–207 (2008).
Khalil, M. M., Tremoleda, J. L., Bayomy, T. B. & Gsell, W. Molecular SPECT imaging: an overview. Int. J. Mol. Imaging 2011, 796025 (2011).
Modlin, I. M., Kidd, M., Latich, I., Zikusoka, M. N. & Shapiro, M. D. Current status of gastrointestinal carcinoids. Gastroenterology 128, 1717–1751 (2005).
Reubi, J. C., Waser, B., Schaer, J. C. & Laissue, J. A. Somatostatin receptor SST1-SST5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur. J. Nucl. Med. 28, 836–846 (2001).
Virgolini, I. et al. In- and Y-DOTA-lanreotide: results and implications of the MAURITIUS trial. Semin. Nucl. Med. 32, 148–155 (2002).
Lebtahi, R. et al. Detection of neuroendocrine tumors: 99mTc-P829 scintigraphy compared with 111In-pentetreotide scintigraphy. J. Nucl. Med. 43, 889–895 (2002).
Koopmans, K. P. et al. Molecular imaging in neuroendocrine tumors: molecular uptake mechanisms and clinical results. Crit. Rev. Oncol. Hematol. 71, 199–213 (2009).
Bombardieri, E. et al. 111In-pentetreotide scintigraphy: procedure guidelines for tumour imaging. Eur. J. Nucl. Med. Mol. Imaging 37, 1441–1448 (2010).
Balon, H. R. et al. The SNM practice guideline for somatostatin receptor scintigraphy 2.0. J. Nucl. Med. Technol. 39, 317–324 (2011).
Modlin, I. M. & Tang, L. H. Approaches to the diagnosis of gut neuroendocrine tumors: the last word (today). Gastroenterology 112, 583–590 (1997).
Kaplan, E. L. & Lee, C. H. Recent advances in the diagnosis and treatment of insulinomas. Surg. Clin. North Am. 59, 119–129 (1979).
Wild, D. et al. Glucagon-like peptide-1 versus somatostatin receptor targeting reveals 2 distinct forms of malignant insulinomas. J. Nucl. Med. 52, 1073–1078 (2011).
Reubi, J. C. & Waser, B. Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. Eur. J. Nucl. Med. Mol. Imaging 30, 781–793 (2003).
Wild, D., Macke, H., Christ, E., Gloor, B. & Reubi, J. C. Glucagon-like peptide 1-receptor scans to localize occult insulinomas. N. Engl. J. Med. 359, 766–768 (2008).
Christ, E. et al. Glucagon-like peptide-1 receptor imaging for localization of insulinomas. J. Clin. Endocrinol. Metab. 94, 4398–4405 (2009).
Wild, D. et al. 'Running on empty'. Eur. J. Nucl. Med. Mol. Imaging 37, 1439–1440 (2010).
Lu, S. J., Gnanasegaran, G., Buscombe, J. & Navalkissoor, S. Single photon emission computed tomography/computed tomography in the evaluation of neuroendocrine tumours: a review of the literature. Nucl. Med. Commun. 34, 98–107 (2013).
Christenson, J. G., Dairman, W. & Udenfriend, S. On the identity of DOPA decarboxylase and 5-hydroxytryptophan decarboxylase (immunological titration-aromatic L-amino acid decarboxylase-serotonin--dopamine-norepinephrine). Proc. Natl Acad. Sci. USA 69, 343–347 (1972).
Orlefors, H. et al. Carbidopa pretreatment improves image interpretation and visualisation of carcinoid tumours with 11C5-hydroxytryptophan positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 33, 60–65 (2006).
National Nuclear Data Center. Nuclear structure and decay data [online], (2013).
Hofland, L. J. & Lamberts, S. W. Somatostatin receptor subtype expression in human tumors. Ann. Oncol. 12 (Suppl. 2), S31–S36 (2001).
Eriksson, B. et al. Developments in PET for the detection of endocrine tumours. Best Pract. Res. Clin. Endocrinol. Metab. 19, 311–324 (2005).
Timmers, H. J. et al. The effects of carbidopa on uptake of 6-18F-Fluoro-L-DOPA in PET of pheochromocytoma and extraadrenal abdominal paraganglioma. J. Nucl. Med. 48, 1599–1606 (2007).
Kauhanen, S., Seppänen, M. & Nuutila, P. Premedication with carbidopa masks positive finding of insulinoma and β-cell hyperplasia in [18F]-dihydroxy-phenyl-alanine positron emission tomography. J. Clin. Oncol. 26, 5307–5308 (2008).
Antunes, P. et al. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur. J. Nucl. Med. Mol. Imaging 34, 982–993 (2007).
Treglia, G., Castaldi, P., Rindi, G., Giordano, A. & Rufini, V. Diagnostic performance of Gallium-68 somatostatin receptor PET and PET/CT in patients with thoracic and gastroenteropancreatic neuroendocrine tumours: a meta-analysis. Endocrine 42, 80–87 (2012).
Freudenberg, L. S., Rosenbaum, S. J., Beyer, T., Bockisch, A. & Antoch, G. PET versus PET/CT dual-modality imaging in evaluation of lung cancer. Radiol. Clin. North Am. 45, 639–644 (2007).
Breeman, W. A. et al. 68Ga-labeled DOTA-peptides and (68)Ga-labeled radiopharmaceuticals for positron emission tomography: current status of research, clinical applications, and future perspectives. Semin. Nucl. Med. 41, 314–321 (2011).
Ambrosini, V., Campana, D., Tomassetti, P. & Fanti, S. 68Ga-labelled peptides for diagnosis of gastroenteropancreatic NET. Eur. J. Nucl. Med. Mol. Imaging 39, S52–S60 (2012).
Virgolini, I. et al. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur. J. Nucl. Med. Mol. Imaging 37, 2004–2010 (2010).
Ambrosini, V. et al. Comparison between 68Ga-DOTA-NOC and 18F-DOPA PET for the detection of gastro-entero-pancreatic and lung neuro-endocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 35, 1431–1438 (2008).
Haug, A. et al. Intraindividual comparison of 68Ga-DOTA-TATE and 18F-DOPA PET in patients with well-differentiated metastatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 36, 765–770 (2009).
Putzer, D. et al. Comparison of (68)Ga-DOTA-Tyr(3)-octreotide and 18)F-fluoro-L-dihydroxyphenylalanine positron emission tomography in neuroendocrine tumor patients. Q. J. Nucl. Med. Mol. Imaging 54, 68–75 (2010).
Binderup, T. et al. Gene expression of glucose transporter 1 (GLUT1), hexokinase 1 and hexokinase 2 in gastroenteropancreatic neuroendocrine tumors: correlation with F18--fluorodeoxyglucose positron emission tomography and cellular proliferation. Diagnostics 3, 372–384 (2013).
Pasquali, C. et al. Neuroendocrine tumor imaging: can 18F-fluorodeoxyglucose positron emission tomography detect tumors with poor prognosis and aggressive behavior? World J. Surg. 22, 588–592 (1998).
Binderup, T. et al. Functional imaging of neuroendocrine tumors: a head-to-head comparison of somatostatin receptor scintigraphy, 123I-MIBG scintigraphy, and 18F-FDG PET. J. Nucl. Med. 51, 704–712 (2010).
Adams, S. et al. Metabolic (PET) and receptor (SPET) imaging of well- and less well-differentiated tumours: comparison with the expression of the Ki-67 antigen. Nucl. Med. Commun. 19, 641–647 (1998).
Ambrosini, V., Fani, M., Fanti, S., Forrer, F. & Maecke, H. R. Radiopeptide imaging and therapy in Europe. J. Nucl. Med. 52, 42S–55S (2011).
Teunissen, J. J., Kwekkeboom, D. J., Valkema, R. & Krenning, E. P. Nuclear medicine techniques for the imaging and treatment of neuroendocrine tumours. Endocr. Relat. Cancer 18, S27–S51 (2011).
Hofmann, M. et al. Biokinetics and imaging with the somatostatin receptor PET radioligand (68)Ga-DOTATOC: preliminary data. Eur. J. Nucl. Med. 28, 1751–1757 (2001).
Kowalski, J. et al. Evaluation of positron emission tomography imaging using [68Ga]-DOTA-D Phe(1)-Tyr(3)-Octreotide in comparison to [111In]-DTPAOC SPECT. First results in patients with neuroendocrine tumors. Mol. Imaging Biol. 5, 42–48 (2003).
Buchmann, I. et al. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 34, 1617–1626 (2007).
Srirajaskanthan, R. et al. The role of 68Ga-DOTATATE PET in patients with neuroendocrine tumors and negative or equivocal findings on 111In-DTPA-octreotide scintigraphy. J. Nucl. Med. 51, 875–882 (2010).
Krausz, Y. et al. 68Ga-DOTA-NOC PET/CT imaging of neuroendocrine tumors: comparison with ¹¹¹In-DTPA-octreotide (OctreoScan®). Mol. Imaging Biol. 13, 583–593 (2011).
Hofman, M. S. et al. High management impact of Ga-68 DOTATATE (GaTate) PET/CT for imaging neuroendocrine and other somatostatin expressing tumours. J. Med. Imaging Radiat. Oncol. 56, 40–47 (2012).
Gabriel, M. et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J. Nucl. Med. 48, 508–518 (2007).
Wild, D. et al. Comparison of 68Ga-DOTANOC and 68Ga-DOTATATE PET/CT within patients with gastroenteropancreatic neuroendocrine tumors. J. Nucl. Med. 54, 364–372 (2013).
Poeppel, T. D. et al. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J. Nucl. Med. 52, 1864–1870 (2011).
Kabasakal, L. et al. Comparison of 68Ga-DOTATATE and 68Ga-DOTANOC PET/CT imaging in the same patient group with neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 39, 1271–1277 (2012).
Koopmans, K. P. et al. Staging of carcinoid tumours with 18F-DOPA PET: a prospective, diagnostic accuracy study. Lancet Oncol. 7, 728–734 (2006).
Koopmans, K. P. et al. Improved staging of patients with carcinoid and islet cell tumors with 18F-dihydroxy-phenyl-alanine and 11C-5-hydroxy-tryptophan positron emission tomography. J. Clin. Oncol. 26, 1489–1495 (2008).
Schiesser, M. et al. Value of combined 6-[18F]fluorodihydroxyphenylalanine PET/CT for imaging of neuroendocrine tumours. Br. J. Surg. 97, 691–697 (2010).
Montravers, F. et al. Can fluorodihydroxyphenylalanine PET replace somatostatin receptor scintigraphy in patients with digestive endocrine tumors? J. Nucl. Med. 47, 1455–1462 (2006).
Yakemchuk, V. N. et al. PET/CT using 18F-FDOPA provides improved staging of carcinoid tumor patients in a Canadian setting. Nucl. Med. Commun. 33, 322–330 (2012).
Becherer, A. et al. Imaging of advanced neuroendocrine tumors with 18)F-FDOPA PET. J. Nucl. Med. 45, 1161–1167 (2004).
Balogova, S. et al. 18F-Fluorodihydroxyphenylalanine vs other radiopharmaceuticals for imaging neuroendocrine tumours according to their type. Eur. J. Nucl. Med. Mol. Imaging 40, 943–966 (2013).
Orlefors, H. et al. Whole-body 11)C-5-hydroxytryptophan positron emission tomography as a universal imaging technique for neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and computed tomography. J. Clin. Endocrinol. Metab. 90, 3392–3400 (2005).
Abgral, R. et al. Performance of 18Fluorodeoxyglucose-positron emission tomography and somatostatin receptor scintigraphy for high Ki67 (≥10%) well-differentiated endocrine carcinoma staging. J. Clin. Endocrinol. Metab. 96, 665–671 (2011).
Salazar, R. et al. ENETS 2011 consensus guidelines for the management of patients with digestive neuroendocrine tumors: an update. Neuroendocrinology 95, 71–73 (2012).
Kvols, L. K. et al. The North American Neuroendocrine Tumor Society (NANETS) guidelines: mission, goals, and process. Pancreas 39, 705–706 (2010).
Kwekkeboom, D. J. et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: somatostatin receptor imaging with 111In-pentetreotide. Neuroendocrinology 90, 184–189 (2009).
Ito, T., Igarashi, H. & Jensen, R. T. Pancreatic neuroendocrine tumors: clinical features, diagnosis and medical treatment: advances. Best Pract. Res. Clin. Gastroenterol. 26, 737–753 (2012).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Strosberg, J. R. Systemic treatment of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): current approaches and future options. Endocr. Pract. http://dx.doi.org/10.4158/EP13262.RA.
Sundin, A. & Rockall, A. Therapeutic monitoring of gastroenteropancreatic neuroendocrine tumors: the challenges ahead. Neuroendocrinology 96, 261–271 (2012).
Choi, H. et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J. Clin. Oncol. 25, 1753–1759 (2007).
Young, H. et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur. J. Cancer 35, 1773–1782 (1999).
Wahl, R. L., Jacene, H., Kasamon, Y. & Lodge, M. A. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J. Nucl. Med. 50, 122S–150S (2009).
Kwekkeboom, D. J. et al. Radiolabeled somatostatin analog [177Lu-DOTA0, Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J. Clin. Oncol. 23, 2754–2762 (2005).
Haug, A. R. et al. 68Ga-DOTATATE PET/CT for the early prediction of response to somatostatin receptor-mediated radionuclide therapy in patients with well-differentiated neuroendocrine tumors. J. Nucl. Med. 51, 1349–1356 (2010).
Gabriel, M. et al. 68Ga-DOTA-Tyr3-octreotide PET for assessing response to somatostatin-receptor-mediated radionuclide therapy. J. Nucl. Med. 50, 1427–1434 (2009).
Velikyan, I. et al. In vivo binding of [68Ga]-DOTATOC to somatostatin receptors in neuroendocrine tumours--impact of peptide mass. Nucl. Med. Biol. 37, 265–275 (2010).
Yao, J. C. et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 364, 514–523 (2011).
Binderup, T., Knigge, U., Loft, A., Federspiel, B. & Kjaer, A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin. Cancer Res. 16, 978–985 (2010).
Severi, S. et al. Role of 18FDG PET/CT in patients treated with 177Lu-DOTATATE for advanced differentiated neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 40, 881–888 (2013).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
E.P. Krenning and D.J. Kwekkeboom are stockholders in Advanced Accelerator Applications (AAA). The other authors declare no competing interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
van Essen, M., Sundin, A., Krenning, E. et al. Neuroendocrine tumours: the role of imaging for diagnosis and therapy. Nat Rev Endocrinol 10, 102–114 (2014). https://doi.org/10.1038/nrendo.2013.246
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2013.246
This article is cited by
-
Rectal neuroendocrine neoplasms: what the radiologists should know
Abdominal Radiology (2022)
-
Radiology of the neuroendocrine neoplasms of the gastrointestinal tract: a comprehensive review
Abdominal Radiology (2021)
-
Evaluation of block-sequential regularized expectation maximization reconstruction of 68Ga-DOTATOC, 18F-fluoride, and 11C-acetate whole-body examinations acquired on a digital time-of-flight PET/CT scanner
EJNMMI Physics (2020)
-
Machine learning applications in imaging analysis for patients with pituitary tumors: a review of the current literature and future directions
Pituitary (2020)