Key Points
-
Obstructive sleep apnoea (OSA) is a very common disorder in the general population, and 50–65% of adult and pediatric patients with OSA are obese
-
Intermittent hypoxaemia during sleep and fragmentation of sleep architecture are the two major constitutive perturbations that characterize OSA
-
In epidemiological studies, OSA is independently associated with metabolic comorbidities, such as the metabolic syndrome, fatty liver disease, adipose tissue dysfunction, insulin resistance and atherosclerosis, particularly when obesity is concurrently present
-
Despite divergent phenotypic effects on adipose tissue, both intermittent hypoxaemia during sleep and sleep fragmentation have been mechanistically linked to altered metabolic phenotypes in preclinical studies performed in rodent models
-
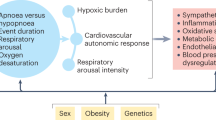
Systemic and organ-specific inflammation, oxidative stress and autonomic nervous system imbalance probably contribute to OSA-associated metabolic dysfunction; other mechanisms, including gut microbiota dysbiosis and endoplasmic reticulum stress, are under investigation
-
Interventional trials in which patients with OSA were effectively treated reveal variable subsequent improvements in metabolic morbidity, which suggests complex interactions between alterations in sleep and oxygenation and obesity
Abstract
Obstructive sleep apnoea (OSA) is a very common disorder that affects 10–25% of the general population. In the past two decades, OSA has emerged as a cardiometabolic risk factor in both paediatric and adult populations. OSA-induced metabolic perturbations include dyslipidaemia, atherogenesis, liver dysfunction and abnormal glucose metabolism. The mainstay of treatment for OSA is adenotonsillectomy in children and continuous positive airway pressure therapy in adults. Although these therapies are effective at resolving the sleep-disordered breathing component of OSA, they do not always produce beneficial effects on metabolic function. Thus, a deeper understanding of the underlying mechanisms by which OSA influences metabolic dysfunction might yield improved therapeutic approaches and outcomes. In this Review, we summarize the evidence obtained from animal models and studies of patients with OSA of potential mechanistic pathways linking the hallmarks of OSA (intermittent hypoxia and sleep fragmentation) with metabolic dysfunction. Special emphasis is given to adipose tissue dysfunction induced by sleep apnoea, which bears a striking resemblance to adipose dysfunction resulting from obesity. In addition, important gaps in current knowledge and promising lines of future investigation are identified.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Peppard, P. E. et al. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 177, 1006–1014 (2013).
Redline, S. et al. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am. J. Respir. Crit. Care Med. 189, 335–344 (2014).
Sanchez-de-la-Torre, M., Campos-Rodriguez, F. & Barbe, F. Obstructive sleep apnoea and cardiovascular disease. Lancet Respir. Med. 1, 61–72 (2013).
Goncalves, M. et al. Sleepiness at the wheel across Europe: a survey of 19 countries. J. Sleep Res. 24, 242–253 (2015).
Lacasse, Y., Godbout, C. & Series, F. Health-related quality of life in obstructive sleep apnoea. Eur. Respir. J. 19, 499–503 (2002).
Young, T. et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 31, 1071–1078 (2008).
Martinez-Ceron, E., Fernandez-Navarro, I. & Garcia-Rio, F. Effects of continuous positive airway pressure treatment on glucose metabolism in patients with obstructive sleep apnea. Sleep Med. Rev. 25, 121–130 (2015).
Hakim, F., Kheirandish-Gozal, L. & Gozal, D. Obesity and altered sleep: a pathway to metabolic derangements in children? Semin. Pediatr. Neurol. 22, 77–85 (2015).
Koren, D., O'Sullivan, K. L. & Mokhlesi, B. Metabolic and glycemic sequelae of sleep disturbances in children and adults. Curr. Diab. Rep. 15, 562 (2015).
Tanno, S. et al. Sleep-related intermittent hypoxemia and glucose intolerance: a community-based study. Sleep Med. 15, 1212–1218 (2014).
Hendricks, J. C. et al. The English bulldog: a natural model of sleep-disordered breathing. J. Appl. Physiol. (1985) 63, 1344–1350 (1987).
Brennick, M. J. et al. Tongue fat infiltration in obese versus lean Zucker rats. Sleep 37, 1095–1102 (2014).
Chopra, S., Polotsky, V. Y. & Jun, J. C. Sleep apnea research in animals: past, present, and future. Am. J. Respir. Cell. Mol. Biol. http://dx.doi.org/10.1165/rcmb.2015-0218TR, (2015).
Carreras, A., Wang, Y. & Gozal, D. in Encyclopedia of Sleep (ed. Kushida, C.) 184–191 (Academic Press, 2013).
Almendros, I., Wang, Y. & Gozal, D. The polymorphic and contradictory aspects of intermittent hypoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 307, L129–L140 (2014).
Tagaito, Y. et al. A model of sleep-disordered breathing in the C57BL/6J mouse. J. Appl. Physiol. (1985) 91, 2758–2766 (2001).
Unnikrishnan, D., Jun, J. & Polotsky, V. Inflammation in sleep apnea: an update. Rev. Endocr. Metab. Disord. 16, 25–34 (2015).
Schulz, R. et al. Arterial hypertension in a murine model of sleep apnea: role of NADPH oxidase 2. J. Hypertens. 32, 300–305 (2014).
Drager, L. F. et al. Chronic intermittent hypoxia induces atherosclerosis via activation of adipose angiopoietin-like 4. Am. J. Respir. Crit. Care Med. 188, 240–248 (2013).
Iiyori, N. et al. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am. J. Respir. Crit. Care Med. 175, 851–857 (2007).
Drager, L. F., Jun, J. C. & Polotsky, V. Y. Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract. Res. Clin. Endocrinol. Metab. 24, 843–851 (2010).
Louis, M. & Punjabi, N. M. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J. Appl. Physiol. (1985) 106, 1538–1544 (2009).
Tamisier, R. et al. 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur. Respir. J. 37, 119–128 (2011).
Stamatakis, K. A. & Punjabi, N. M. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest 137, 95–101 (2010).
Trzepizur, W. et al. Independent association between nocturnal intermittent hypoxemia and metabolic dyslipidemia. Chest 143, 1584–1589 (2013).
Newman, A. B. et al. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am. J. Epidemiol. 154, 50–59 (2001).
Wu, W. T. et al. The association between obstructive sleep apnea and metabolic markers and lipid profiles. PLoS ONE 10, e0130279 (2015).
Jun, J. C. et al. Effects of sleep apnea on nocturnal free fatty acids in subjects with heart failure. Sleep 34, 1207–1213 (2011).
Barcelo, A. et al. Free fatty acids and the metabolic syndrome in patients with obstructive sleep apnoea. Eur. Respir. J. 37, 1418–1423 (2011).
Robinson, G. V., Pepperell, J. C., Segal, H. C., Davies, R. J. & Stradling, J. R. Circulating cardiovascular risk factors in obstructive sleep apnoea: data from randomised controlled trials. Thorax 59, 777–782 (2004).
Chirinos, J. A. et al. CPAP, weight loss, or both for obstructive sleep apnea. N. Engl. J. Med. 370, 2265–2275 (2014).
Rebelo, S., Drummond, M. & Marques, J. A. Lipid profile after long-term APAP in OSA patients. Sleep Breath. 19, 931–937 (2015).
Phillips, C. L. et al. Continuous positive airway pressure reduces postprandial lipidemia in obstructive sleep apnea: a randomized, placebo-controlled crossover trial. Am. J. Respir. Crit. Care Med. 184, 355–361 (2011).
Sivam, S. et al. Effects of 8 weeks of CPAP on lipid-based oxidative markers in obstructive sleep apnea: a randomized trial. J. Sleep Res. 24, 339–345 (2015).
Nadeem, R. et al. Effect of CPAP treatment for obstructive sleep apnea hypopnea syndrome on lipid profile: a meta-regression analysis. J. Clin. Sleep Med. 10, 1295–1302 (2014).
Jullian-Desayes, I. et al. Impact of obstructive sleep apnea treatment by continuous positive airway pressure on cardiometabolic biomarkers: a systematic review from sham CPAP randomized controlled trials. Sleep Med. Rev. 21, 23–38 (2015).
Tauman, R., O'Brien, L. M., Ivanenko, A. & Gozal, D. Obesity rather than severity of sleep-disordered breathing as the major determinant of insulin resistance and altered lipidemia in snoring children. Pediatrics 116, E66–E73 (2005).
Koren, D., Gozal, D., Bhattacharjee, R., Philby, M. & Kheirandish-Gozal, L. Impact of adenotonsillectomy on insulin resistance and lipoprotein profile in nonobese and obese children. Chest http://dx.doi.org/10.1378/chest.15-1543, (2015).
Van Hoorenbeeck, K. et al. Metabolic disregulation in obese adolescents with sleep-disordered breathing before and after weight loss. Obesity (Silver Spring) 21, 1446–1450 (2013).
Quante, M. et al. The effect of adenotonsillectomy for childhood sleep apnea on cardiometabolic measures. Sleep 38, 1395–1403 (2014).
Ip, M. S. et al. Obstructive sleep apnea is independently associated with insulin resistance. Am. J. Respir. Crit. Care Med. 165, 670–676 (2002).
Pamidi, S. et al. Obstructive sleep apnea in young lean men: impact on insulin sensitivity and secretion. Diabetes Care 35, 2384–2389 (2012).
Punjabi, N. M. et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am. J. Epidemiol. 160, 521–530 (2004).
Parish, J. M., Adam, T. & Facchiano, L. Relationship of metabolic syndrome and obstructive sleep apnea. J. Clin. Sleep Med. 3, 467–472 (2007).
Muraki, I. et al. Nocturnal intermittent hypoxia and the development of type 2 diabetes: the Circulatory Risk in Communities Study (CIRCS). Diabetologia 53, 481–488 (2010).
Tasali, E., Mokhlesi, B. & Van Cauter, E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest 133, 496–506 (2008).
Babu, A. R., Herdegen, J., Fogelfeld, L., Shott, S. & Mazzone, T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch. Intern. Med. 165, 447–452 (2005).
Grimaldi, D., Beccuti, G., Touma, C., Van Cauter, E. & Mokhlesi, B. Association of obstructive sleep apnea in rapid eye movement sleep with reduced glycemic control in type 2 diabetes: therapeutic implications. Diabetes Care 37, 355–363 (2014).
Aronsohn, R. S., Whitmore, H., Van Cauter, E. & Tasali, E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am. J. Respir. Crit. Care Med. 181, 507–513 (2010).
Foster, G. D. et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care 32, 1017–1019 (2009).
Mokhlesi, B., Ham, S. A. & Gozal, D. The effect of sex and age on the comorbidity burden of OSA: an observational analysis from a large nationwide US health claims database. Eur. Respir. J. http://dx.doi.org/10.1183/13993003.01618-2015 (2016).
Harsch, I. A. et al. The effect of continuous positive airway pressure treatment on insulin sensitivity in patients with obstructive sleep apnoea syndrome and type 2 diabetes. Respiration 71, 252–259 (2004).
Harsch, I. A. et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 169, 156–162 (2004).
Guest, J. F., Panca, M., Sladkevicius, E., Taheri, S. & Stradling, J. Clinical outcomes and cost-effectiveness of continuous positive airway pressure to manage obstructive sleep apnea in patients with type 2 diabetes in the UK. Diabetes Care 37, 1263–1271 (2014).
West, S. D., Nicoll, D. J., Wallace, T. M., Matthews, D. R. & Stradling, J. R. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax 62, 969–974 (2007).
Hoyos, C. M. et al. Cardiometabolic changes after continuous positive airway pressure for obstructive sleep apnoea: a randomised sham-controlled study. Thorax 67, 1081–1089 (2012).
Lam, J. C., Tan, K. C., Lai, A. Y., Lam, D. C. & Ip, M. S. Increased serum levels of advanced glycation end-products is associated with severity of sleep disordered breathing but not insulin sensitivity in non-diabetic men with obstructive sleep apnoea. Sleep Med. 13, 15–20 (2012).
Weinstock, T. G. et al. A controlled trial of CPAP therapy on metabolic control in individuals with impaired glucose tolerance and sleep apnea. Sleep 35, 617–625B (2012).
Iftikhar, I. H., Hoyos, C. M., Phillips, C. L. & Magalang, U. J. Meta-analyses of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J. Clin. Sleep Med. 11, 475–485 (2015).
Schahin, S. P. et al. Long-term improvement of insulin sensitivity during CPAP therapy in the obstructive sleep apnoea syndrome. Med. Sci. Monit. 14, CR117–CR121 (2008).
Pamidi, S. et al. Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes. A randomized controlled trial. Am. J. Respir. Crit. Care Med. 192, 96–105 (2015).
Gozal, D., Capdevila, O. S. & Kheirandish-Gozal, L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am. J. Respir. Crit. Care Med. 177, 1142–1149 (2008).
Shamsuzzaman, A., Szczesniak, R. D., Fenchel, M. C. & Amin, R. S. Glucose, insulin, and insulin resistance in normal-weight, overweight and obese children with obstructive sleep apnea. Obes Res. Clin. Pract. 8, e584–e591 (2014).
Lesser, D. J. et al. Sleep fragmentation and intermittent hypoxemia are associated with decreased insulin sensitivity in obese adolescent Latino males. Pediatr. Res. 72, 293–298 (2012).
Li, J. et al. Chronic intermittent hypoxia upregulates genes of lipid biosynthesis in obese mice. J. Appl. Physiol. (1985) 99, 1643–1648 (2005).
Li, J. G. et al. Intermittent hypoxia induces hyperlipidemia in lean mice. Circul. Res. 97, 698–706 (2005).
Li, J. et al. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J. Appl. Physiol. (1985) 102, 557–563 (2007).
Drager, L. F. et al. Intermittent hypoxia inhibits clearance of triglyceride-rich lipoproteins and inactivates adipose lipoprotein lipase in a mouse model of sleep apnoea. Eur. Heart J. 33, 783–790 (2012).
Drager, L. F. et al. Intermittent hypoxia exacerbates metabolic effects of diet-induced obesity. Obesity (Silver Spring) 19, 2167–2174 (2011).
Van Noolen, L. et al. Docosahexaenoic acid supplementation modifies fatty acid incorporation in tissues and prevents hypoxia induced-atherosclerosis progression in apolipoprotein-E deficient mice. Prostaglandins Leukot. Essent. Fatty Acids 91, 111–117 (2014).
Ferrarini, A. et al. Fingerprinting-based metabolomic approach with LC-MS to sleep apnea and hypopnea syndrome: a pilot study. Electrophoresis 34, 2873–2881 (2013).
Carreras, A. et al. Resveratrol attenuates intermittent hypoxia-induced macrophage migration to visceral white adipose tissue and insulin resistance in male mice. Endocrinology 156, 437–443 (2015).
Nachalon, Y., Lowenthal, N., Greenberg-Dotan, S. & Goldbart, A. D. Inflammation and growth in young children with obstructive sleep apnea syndrome before and after adenotonsillectomy. Mediators Inflamm. 2014, 146893 (2014).
Ogretmenoglu, O., Suslu, A. E., Yucel, O. T., Onerci, T. M. & Sahin, A. Body fat composition: a predictive factor for obstructive sleep apnea. Laryngoscope 115, 1493–1498 (2005).
Garcia-Fuentes, E. et al. Hypoxia is associated with a lower expression of genes involved in lipogenesis in visceral adipose tissue. J. Transl. Med. 13, 373 (2015).
Hodson, L. Adipose tissue oxygenation: effects on metabolic function. Adipocyte 3, 75–80 (2014).
Kayser, B. & Verges, S. Hypoxia, energy balance and obesity: from pathophysiological mechanisms to new treatment strategies. Obes. Rev. 14, 579–592 (2013).
Pasarica, M. et al. Reduced oxygenation in human obese adipose tissue is associated with impaired insulin suppression of lipolysis. J. Clin. Endocrinol. Metab. 95, 4052–4055 (2010).
Trayhurn, P. Hypoxia and adipocyte physiology: implications for adipose tissue dysfunction in obesity. Annu. Rev. Nutr. 34, 207–236 (2014).
Pasarica, M. et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 58, 718–725 (2009).
Fujisaka, S. et al. Adipose tissue hypoxia induces inflammatory M1 polarity of macrophages in an HIF-1α-dependent and HIF-1α-independent manner in obese mice. Diabetologia 56, 1403–1412 (2013).
Kim, D. H., Gutierrez-Aguilar, R., Kim, H. J., Woods, S. C. & Seeley, R. J. Increased adipose tissue hypoxia and capacity for angiogenesis and inflammation in young diet-sensitive C57 mice compared with diet-resistant FVB mice. Int. J. Obes. (Lond.) 37, 853–860 (2013).
Hodson, L., Humphreys, S. M., Karpe, F. & Frayn, K. N. Metabolic signatures of human adipose tissue hypoxia in obesity. Diabetes 62, 1417–1425 (2013).
Matsuura, H. et al. Prolyl hydroxylase domain protein 2 plays a critical role in diet-induced obesity and glucose intolerance. Circulation 127, 2078–2087 (2013).
Rahtu-Korpela, L. et al. HIF prolyl 4-hydroxylase-2 inhibition improves glucose and lipid metabolism and protects against obesity and metabolic dysfunction. Diabetes 63, 3324–3333 (2014).
Choe, S. S. et al. Macrophage HIF-2α ameliorates adipose tissue inflammation and insulin resistance in obesity. Diabetes 63, 3359–3371 (2014).
Jiang, C. et al. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes 60, 2484–2495 (2011).
Lee, Y. S. et al. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell 157, 1339–1352 (2014).
Scalia, R. The microcirculation in adipose tissue inflammation. Rev. Endocr. Metab. Disord. 14, 69–76 (2013).
Shimizu, I. et al. Vascular rarefaction mediates whitening of brown fat in obesity. J. Clin. Invest. 124, 2099–2112 (2014).
Villela, N. R., Kramer-Aguiar, L. G., Bottino, D. A., Wiernsperger, N. & Bouskela, E. Metabolic disturbances linked to obesity: the role of impaired tissue perfusion. Arq. Bras. Endocrinol. Metabol. 53, 238–245 (2009).
Michailidou, Z. et al. Increased angiogenesis protects against adipose hypoxia and fibrosis in metabolic disease-resistant 11β-hydroxysteroid dehydrogenase type 1 (HSD1)-deficient mice. J. Biol. Chem. 287, 4188–4197 (2012).
Farb, M. G. et al. Arteriolar function in visceral adipose tissue is impaired in human obesity. Arterioscler. Thromb. Vasc. Biol. 32, 467–473 (2012).
Hill, A. A., Reid Bolus, W. & Hasty, A. H. A decade of progress in adipose tissue macrophage biology. Immunol. Rev. 262, 134–152 (2014).
Martinez-Santibanez, G. & Lumeng, C. N. Macrophages and the regulation of adipose tissue remodeling. Annu. Rev. Nutr. 34, 57–76 (2014).
Schipper, H. S., Prakken, B., Kalkhoven, E. & Boes, M. Adipose tissue-resident immune cells: key players in immunometabolism. Trends Endocrinol. Metab. 23, 407–415 (2012).
Mathis, D. Immunological goings-on in visceral adipose tissue. Cell Metab. 17, 851–859 (2013).
Ghigliotti, G. et al. Adipose tissue immune response: novel triggers and consequences for chronic inflammatory conditions. Inflammation 37, 1337–1353 (2014).
O'Rourke, R. W. et al. Systemic NK cell ablation attenuates intra-abdominal adipose tissue macrophage infiltration in murine obesity. Obesity (Silver Spring) 22, 2109–2114 (2014).
Morris, D. L. et al. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes 62, 2762–2772 (2013).
Cipolletta, D. Adipose tissue-resident regulatory T cells: phenotypic specialization, functions and therapeutic potential. Immunology 142, 517–525 (2014).
Lee, J. H., Gao, Z. & Ye, J. Regulation of 11β-HSD1 expression during adipose tissue expansion by hypoxia through different activities of NF-κB and HIF-1α. Am. J. Physiol. Endocrinol. Metab. 304, E1035–1041 (2013).
Cho, K. W. et al. An MHC II-dependent activation loop between adipose tissue macrophages and CD4+ T cells controls obesity-induced inflammation. Cell Rep. 9, 605–617 (2014).
Poulain, L. et al. Visceral white fat remodelling contributes to intermittent hypoxia-induced atherogenesis. Eur. Respir. J. 43, 513–522 (2014).
Poulain, L., Richard, V., Levy, P., Dematteis, M. & Arnaud, C. Toll-like receptor-4 mediated inflammation is involved in the cardiometabolic alterations induced by intermittent hypoxia. Mediators Inflamm. 2015, 620258 (2015).
Carreras, A. et al. Metabolic effects of intermittent hypoxia in mice: steady versus high-frequency applied hypoxia daily during the rest period. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303, R700–R709 (2012).
Lee, E. J. et al. Time-dependent changes in glucose and insulin regulation during intermittent hypoxia and continuous hypoxia. Eur. J. Appl. Physiol. 113, 467–478 (2013).
Olea, E. et al. Intermittent hypoxia and diet-induced obesity: effects on oxidative status, sympathetic tone, plasma glucose and insulin levels, and arterial pressure. J. Appl. Physiol. (1985) 117, 706–719 (2014).
van den Borst, B. et al. Characterization of the inflammatory and metabolic profile of adipose tissue in a mouse model of chronic hypoxia. J. Appl. Physiol. (1985) 114, 1619–1628 (2013).
Jun, J. C. et al. Thermoneutrality modifies the impact of hypoxia on lipid metabolism. Am. J. Physiol. Endocrinol. Metab. 304, E424–E435 (2013).
Jun, J. C. et al. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am. J. Physiol. Endocrinol. Metab. 303, E377–E388 (2012).
Mesarwi, O. A., Sharma, E. V., Jun, J. C. & Polotsky, V. Y. Metabolic dysfunction in obstructive sleep apnea: a critical examination of underlying mechanisms. Sleep Biol. Rhythms 13, 2–17 (2015).
Gharib, S. A. et al. Intermittent hypoxia activates temporally coordinated transcriptional programs in visceral adipose tissue. J. Mol. Med. (Berl.) 90, 435–445 (2012).
He, Q. et al. Effects of varying degrees of intermittent hypoxia on proinflammatory cytokines and adipokines in rats and 3T3-L1 adipocytes. PLoS ONE 9, e86326 (2014).
Fiori, C. Z. et al. Downregulation of uncoupling protein-1 mRNA expression and hypoadiponectinemia in a mouse model of sleep apnea. Sleep Breath. 18, 541–548 (2014).
Reinke, C., Bevans-Fonti, S., Drager, L. F., Shin, M. K. & Polotsky, V. Y. Effects of different acute hypoxic regimens on tissue oxygen profiles and metabolic outcomes. J. Appl. Physiol. (1985) 111, 881–890 (2011).
Zuo, L., Pannell, B. K., Re, A. T., Best, T. M. & Wagner, P. D. Po2 cycling protects diaphragm function during reoxygenation via ROS, Akt, ERK, and mitochondrial channels. Am. J. Physiol. Cell Physiol. 309, C759–C766 (2015).
Nair, D., Dayyat, E. A., Zhang, S. X., Wang, Y. & Gozal, D. Intermittent hypoxia-induced cognitive deficits are mediated by NADPH oxidase activity in a murine model of sleep apnea. PLoS ONE 6, e19847 (2011).
Kumar, G. K., Peng, Y. J., Nanduri, J. & Prabhakar, N. R. Carotid body chemoreflex mediates intermittent hypoxia-induced oxidative stress in the adrenal medulla. Adv. Exp. Med. Biol. 860, 195–199 (2015).
Lin, M. et al. Structural remodeling of nucleus ambiguus projections to cardiac ganglia following chronic intermittent hypoxia in C57BL/6J mice. J. Comp. Neurol. 509, 103–117 (2008).
Gu, H. et al. Selective impairment of central mediation of baroreflex in anesthetized young adult Fischer 344 rats after chronic intermittent hypoxia. Am. J. Physiol. Heart Circ. Physiol. 293, H2809–H2818 (2007).
Chalacheva, P., Thum, J., Yokoe, T., O'Donnell, C. P. & Khoo, M. C. Development of autonomic dysfunction with intermittent hypoxia in a lean murine model. Respir. Physiol. Neurobiol. 188, 143–151 (2013).
Zeng, W. et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 163, 84–94 (2015).
Delarue, J. & Magnan, C. Free fatty acids and insulin resistance. Curr. Opin. Clin. Nutr. Metab. Care 10, 142–148 (2007).
Semenza, G. L. & Prabhakar, N. R. Neural regulation of hypoxia-inducible factors and redox state drives the pathogenesis of hypertension in a rodent model of sleep apnea. 119, 1152–1156 (2015).
Saito, Y. et al. Loss of SDHB elevates catecholamine synthesis and secretion depending on ROS production & HIF Stabilization. Neurochem. Res. http://dx.doi.org/10.1007/s11064-015-1738-3 (2015).
Jacintho, J. D. & Kovacic, P. Neurotransmission and neurotoxicity by nitric oxide, catecholamines, and glutamate: unifying themes of reactive oxygen species and electron transfer. Curr. Med. Chem. 10, 2693–2703 (2003).
Jun, J. C. et al. Intermittent hypoxia-induced glucose intolerance is abolished by α-adrenergic blockade or adrenal medullectomy. Am. J. Physiol. Endocrinol. Metab. 307, E1073–E1083 (2014).
Shin, M. K. et al. Carotid body denervation prevents fasting hyperglycemia during chronic intermittent hypoxia. J. Appl. Physiol. (1985) 117, 765–776 (2014).
Haneklaus, M. & O'Neill, L. A. NLRP3 at the interface of metabolism and inflammation. Immunol. Rev. 265, 53–62 (2015).
Lagouge, M. et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127, 1109–1122 (2006).
Wang, Q. et al. Resveratrol attenuates intermittent hypoxia-induced insulin resistance in rats: involvement of Sirtuin 1 and the phosphatidylinositol-4,5-bisphosphate 3-kinase/AKT pathway. Mol. Med. Rep. 11, 151–158 (2015).
Corey, K. E. et al. Obstructive sleep apnea is associated with nonalcoholic steatohepatitis and advanced liver histology. Dig. Dis. Sci. 60, 2523–2528 (2015).
Kheirandish-Gozal, L., Sans Capdevila, O., Kheirandish, E. & Gozal, D. Elevated serum aminotransferase levels in children at risk for obstructive sleep apnea. Chest 133, 92–99 (2008).
Savransky, V. et al. Chronic intermittent hypoxia causes hepatitis in a mouse model of diet-induced fatty liver. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G871–877 (2007).
Savransky, V. et al. Chronic intermittent hypoxia predisposes to liver injury. Hepatology 45, 1007–1013 (2007).
Polak, J. et al. Intermittent hypoxia impairs glucose homeostasis in C57BL6/J mice: partial improvement with cessation of the exposure. Sleep 36, 1483–1490 (2013).
Browning, J. D. & Horton, J. D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 114, 147–152 (2004).
da Rosa, D. P. et al. Antioxidants inhibit the inflammatory and apoptotic processes in an intermittent hypoxia model of sleep apnea. Inflamm. Res. 64, 21–29 (2015).
da Rosa, D. P. et al. Simulating sleep apnea by exposure to intermittent hypoxia induces inflammation in the lung and liver. Mediators Inflamm. 2012, 879419 (2012).
Li, J. et al. Altered metabolic responses to intermittent hypoxia in mice with partial deficiency of hypoxia-inducible factor-1α. Physiol. Genomics 25, 450–457 (2006).
Mirrakhimov, A. E. & Polotsky, V. Y. Obstructive sleep apnea and non-alcoholic fatty liver disease: is the liver another target? Front. Neurol. 3, 149 (2012).
Sherwani, S. I. et al. Intermittent hypoxia exacerbates pancreatic β-cell dysfunction in a mouse model of diabetes mellitus. Sleep 36, 1849–1858 (2013).
Wang, N., Khan, S. A., Prabhakar, N. R. & Nanduri, J. Impaired pancreatic β-cell function by chronic intermittent hypoxia. Exp. Physiol. 98, 1376–1385 (2013).
Pae, E. K. & Kim, G. Insulin production hampered by intermittent hypoxia via impaired zinc homeostasis. PLoS ONE 9, e90192 (2014).
Ota, H. et al. Pancreatic β cell proliferation by intermittent hypoxia via up-regulation of Reg family genes and HGF gene. Life Sci. 93, 664–672 (2013).
Ota, H. et al. Attenuation of glucose-induced insulin secretion by intermittent hypoxia via down-regulation of CD38. Life Sci. 90, 206–211 (2012).
Xu, J., Long, Y. S., Gozal, D. & Epstein, P. N. β-cell death and proliferation after intermittent hypoxia: role of oxidative stress. Free Radic. Biol. Med. 46, 783–790 (2009).
Yokoe, T. et al. Intermittent hypoxia reverses the diurnal glucose rhythm and causes pancreatic beta-cell replication in mice. J. Physiol. 586, 899–911 (2008).
Lo, J. F. et al. Islet preconditioning via multimodal microfluidic modulation of intermittent hypoxia. Anal. Chem. 84, 1987–1993 (2012).
Fang, Y. et al. Intermittent hypoxia-induced rat pancreatic β-cell apoptosis and protective effects of antioxidant intervention. Nutr. Diabetes 4, e131 (2014).
Shin, M. K. et al. The effect of adrenal medullectomy on metabolic responses to chronic intermittent hypoxia. Respir. Physiol. Neurobiol. 203, 60–67 (2014).
Greenhill, C. Gut microbiota: Firmicutes and Bacteroidetes involved in insulin resistance by mediating levels of glucagon-like peptide 1. Nat. Rev. Endocrinol. 11, 254 (2015).
Hartstra, A. V., Bouter, K. E., Backhed, F. & Nieuwdorp, M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care 38, 159–165 (2015).
Parekh, P. J., Arusi, E., Vinik, A. I. & Johnson, D. A. The role and influence of gut microbiota in pathogenesis and management of obesity and metabolic syndrome. Front. Endocrinol. (Lausanne) 5, 47 (2014).
Khan, M. T., Nieuwdorp, M. & Backhed, F. Microbial modulation of insulin sensitivity. Cell Metab. 20, 753–760 (2014).
De Vadder, F. et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156, 84–96 (2014).
Tremaroli, V. & Backhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 489, 242–249 (2012).
Kheirandish-Gozal, L. et al. Lipopolysaccharide-binding protein plasma levels in children: effects of obstructive sleep apnea and obesity. J. Clin. Endocrinol. Metab. 99, 656–663 (2014).
Nobili, V. et al. Altered gut-liver axis and hepatic adiponectin expression in OSAS: novel mediators of liver injury in paediatric non-alcoholic fatty liver. Thorax 70, 769–781 (2015).
Moreno-Indias, I. et al. Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. Eur. Respir. J. 45, 1055–1065 (2015).
Jouvet, D., Vimont, P., Delorme, F. & Jouvet, M. Etude de la privation sélective de la phase paradoxale de sommeil chez le chat. C.R. Soc. Biol. 23, 756–759 (1964).
Tartar, J. L. et al. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur. J. Neurosci. 23, 2739–2748 (2006).
Kaushal, N., Ramesh, V. & Gozal, D. TNF-α and temporal changes in sleep architecture in mice exposed to sleep fragmentation. PLoS ONE 7, e45610 (2012).
Ramesh, V. et al. Disrupted sleep without sleep curtailment induces sleepiness and cognitive dysfunction via the tumor necrosis factor-α pathway. J. Neuroinflamm. 9, 91 (2012).
Kaushal, N., Ramesh, V. & Gozal, D. Human apolipoprotein E4 targeted replacement in mice reveals increased susceptibility to sleep disruption and intermittent hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303, R19–R29 (2012).
Kaushal, N., Nair, D., Gozal, D. & Ramesh, V. Socially isolated mice exhibit a blunted homeostatic sleep response to acute sleep deprivation compared to socially paired mice. Brain Res. 1454, 65–79 (2012).
Nair, D. et al. Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase-dependent pathways in mouse. Am. J. Respir. Crit. Care Med. 184, 1305–1312 (2011).
Leenaars, C. H. et al. Switch-task performance in rats is disturbed by 12 h of sleep deprivation but not by 12 h of sleep fragmentation. Sleep 35, 211–221 (2012).
Tartar, J. L. et al. Sleep fragmentation reduces hippocampal CA1 pyramidal cell excitability and response to adenosine. Neurosci. Lett. 469, 1–5 (2010).
Wang, Y. et al. Chronic sleep fragmentation promotes obesity in young adult mice. Obesity (Silver Spring) 22, 758–762 (2014).
Zhang, S. X. et al. Sleep fragmentation promotes NADPH oxidase 2-mediated adipose tissue inflammation leading to insulin resistance in mice. Int. J. Obes. (Lond.) 38, 619–624 (2014).
Nair, D. et al. Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase–dependent pathways in mouse. Am. J. Respir. Crit. Care Med. 184, 1305–1312 (2011).
Carreras, A. et al. Chronic sleep fragmentation induces endothelial dysfunction and structural vascular changes in mice. Sleep 37, 1817–1824 (2014).
Smith, S. S., Waight, C., Doyle, G., Rossa, K. R. & Sullivan, K. A. Liking for high fat foods in patients with obstructive sleep apnoea. Appetite 78, 185–192 (2014).
Spruyt, K., Sans Capdevila, O., Serpero, L. D., Kheirandish-Gozal, L. & Gozal, D. Dietary and physical activity patterns in children with obstructive sleep apnea. J. Pediatr. 156, 724–730.e3 (2010).
Beebe, D. W., Miller, N., Kirk, S., Daniels, S. R. & Amin, R. The association between obstructive sleep apnea and dietary choices among obese individuals during middle to late childhood. Sleep Med. 12, 797–799 (2011).
Chihara, Y. et al. Among metabolic factors, significance of fasting and postprandial increases in acyl and desacyl ghrelin and the acyl/desacyl ratio in obstructive sleep apnea before and after treatment. J. Clin. Sleep Med. 11, 895–905 (2015).
Ong, C. W., O'Driscoll, D. M., Truby, H., Naughton, M. T. & Hamilton, G. S. The reciprocal interaction between obesity and obstructive sleep apnoea. Sleep Med. Rev. 17, 123–131 (2013).
Hakim, F. et al. Chronic sleep fragmentation during the sleep period induces hypothalamic endoplasmic reticulum stress and PTP1b-mediated leptin resistance in male mice. Sleep 38, 31–40 (2015).
Ozcan, L. et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 9, 35–51 (2009).
Gharib, S. A., Khalyfa, A., Abdelkarim, A., Bhushan, B. & Gozal, D. Integrative miRNA-mRNA profiling of adipose tissue unravels transcriptional circuits induced by sleep fragmentation. PLoS ONE 7, e37669 (2012).
Khalyfa, A. et al. Sleep fragmentation in mice induces nicotinamide adenine dinucleotide phosphate oxidase 2-dependent mobilization, proliferation, and differentiation of adipocyte progenitors in visceral white adipose tissue. Sleep 37, 999–1009 (2014).
Carreras, A. et al. Effect of resveratrol on visceral white adipose tissue inflammation and insulin sensitivity in a mouse model of sleep apnea. Int. J. Obes. (Lond.) 39, 418–423 (2015).
Author information
Authors and Affiliations
Contributions
A.G-H, L.K-G and D.G. researched data for the article, contributed to discussions of the content, wrote the article and reviewed and/or edited the manuscript before submission
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Gileles-Hillel, A., Kheirandish-Gozal, L. & Gozal, D. Biological plausibility linking sleep apnoea and metabolic dysfunction. Nat Rev Endocrinol 12, 290–298 (2016). https://doi.org/10.1038/nrendo.2016.22
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2016.22
This article is cited by
-
Snoring might be a warning sign for metabolic syndrome in nonobese Korean women
Scientific Reports (2023)
-
Effects of heated humidification on positive airway pressure side effects in patients with obstructive sleep apnoea: a meta-analysis
Sleep and Breathing (2023)
-
Intermittent hypoxia-induced METTL3 downregulation facilitates MGLL-mediated lipolysis of adipocytes in OSAS
Cell Death Discovery (2022)
-
Sleep duration, brain structure, and psychiatric and cognitive problems in children
Molecular Psychiatry (2021)
-
The relationship between metabolic syndrome and obstructive sleep apnea syndrome: a nationwide population-based study
Scientific Reports (2021)