Abstract
Systemic inflammation is common in liver failure and its acquisition is a predictor of hepatic encephalopathy severity. New studies provide convincing evidence for a role of neuroinflammation (inflammation of the brain per se) in liver failure; this evidence includes activation of microglia, together with increased synthesis in situ of the proinflammatory cytokines TNF, IL-1β and IL-6. Liver–brain signalling mechanisms in liver failure include: direct effects of systemic proinflammatory molecules, recruitment of monocytes after microglial activation, brain accumulation of ammonia, lactate and manganese, and altered permeability of the blood–brain barrier. Ammonia and cytokines might act synergistically. Existing strategies to reduce ammonia levels (including lactulose, rifaximin and probiotics) have the potential to dampen systemic inflammation, as does albumin dialysis, mild hypothermia and N-acetylcysteine, the latter two agents acting at both peripheral and central sites. Minocycline, an agent with potent central anti-inflammatory properties, reduces neuroinflammation, brain oedema and encephalopathy in liver failure, as does the anti-TNF agent etanercept.
Key Points
-
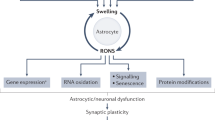
Both acute and chronic liver failure result in neuroinflammation—an inflammatory response in the central nervous system (CNS) characterized by microglial activation and brain accumulation of proinflammatory cytokines
-
Evidence suggests that neuroinflammation contributes to the pathogenesis of the CNS manifestations of acute and chronic liver failure, including hepatic encephalopathy as well as brain oedema and its complications
-
Liver-to-brain proinflammatory signalling in liver failure involves humoral and neural routes; new evidence indicates that monocyte recruitment, changes in levels of ammonia, lactate, manganese and alterations in blood–brain barrier permeability might also have a key role
-
Many currently available therapies used in the treatment of encephalopathy and brain oedema in liver failure (including lactulose, albumin dialysis and mild hypothermia) have the requisite properties to limit systemic and/or neuroinflammation
-
Early investigations found that anti-inflammatory agents (such as ibuprofen, anti-TNF agents and minocycline) are effective in the treatment of the CNS complications of experimental liver failure
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Als-Nielsen, B., Gluud, L. L. & Gluud, C. Nonabsorbable disaccharides for hepatic encephalopathy: systematic review of randomised trials. BMJ 328, 1046 (2004).
Acharya, S. K., Bhatia, V., Sreenivas, V., Khanal, S. & Panda, S. K. Efficacy of L-ornithine L-aspartate in acute liver failure: a double-blind, randomized, placebo-controlled study. Gastroenterology 136, 2159–2168 (2009).
Rolando, N. et al. The systemic inflammatory response syndrome in acute liver failure. Hepatology 32, 734–739 (2000).
Shawcross, D. L., Davies, N. A., Williams, R. & Jalan, R. Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J. Hepatol. 40, 247–254 (2004).
Bémeur, C. & Butterworth, R. F. Liver–brain proinflammatory signalling in acute liver failure: role in the pathogenesis of hepatic encephalopathy and brain edema. Metab. Brain Dis. http://dx.doi.org/10.1007/s11011-012-9361-3.
Vaquero, J. et al. Infection and the progression of hepatic encephalopathy in acute liver failure. Gastroenterology 125, 755–764 (2003).
Odeh, M. Pathogenesis of hepatic encephalopathy: the tumour necrosis factor-α theory. Eur. J. Clin. Invest. 37, 291–304 (2007).
Jiang, W., Desjardins, P. & Butterworth, R. F. Cerebral inflammation contributes to encephalopathy and brain edema in acute liver failure: protective effect of minocycline. J. Neurochem. 109, 485–493 (2009).
Bernal, W., Donaldson, P., Underhill, J., Wendon, J. & Williams, R. Tumor necrosis factor genomic polymorphism and outcome of acetaminophen (paracetamol)-induced acute liver failure. J. Hepatol. 29, 53–59 (1998).
Shawcross, D. L. et al. Infection and systemic inflammation, not ammonia, are associated with Grade 3/4 hepatic encephalopathy, but not mortality in cirrhosis. J. Hepatol. 54, 640–649 (2011).
Jiang, W. et al. Unequivocal evidence for cytokine accumulation in brain in experimental acute liver failure. Hepatology 44 (Suppl.1), 366A (2006).
Wright, G., Shawcross, D., Olde Damink, S. W. & Jalan, R. Brain cytokine flux in acute liver failure and its relationship with intracranial hypertension. Metab. Brain Dis. 22, 375–388 (2007).
Bémeur, C., Vaquero, J., Desjardins, P. & Butterworth, R. F. N-acetylcysteine attenuates cerebral complications of non-acetaminophen-induced acute liver failure in mice: antioxidant and anti-inflammatory mechanisms. Metab. Brain Dis. 25, 241–249 (2010).
Bémeur, C., Qu, H., Desjardins, P. & Butterworth, R. F. IL-1 or TNF receptor gene deletion delays onset of encephalopathy and attenuates brain edema in experimental acute liver failure. Neurochem. Int. 56, 213–215 (2010).
Thrane, V. R. et al. Real-time analysis of microglial activation and motility in hepatic and hyperammonemic encephalopathy. Neuroscience 220, 247–255 (2012).
McMillin, M. et al. Increased neuronal chemokine CCL2/MCP-1 expression is associated with hepatic encephalopathy and contributes to neurological decline. Hepatology 56 (Suppl.1), 958A (2012).
Butterworth, R. F. Hepatic encephalopathy: a central neuroinflammatory disorder? Hepatology 53, 1372–1376 (2011).
D'Mello, C., Le, T. & Swain, M. G. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factor α signaling during peripheral organ inflammation. J. Neurosci. 29, 2089–2102 (2009).
Rodrigo, R. et al. Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology 139, 675–684 (2010).
Cauli, O., Rodrigo, R., Piedrafita, B., Boix, J. & Felipo, V. Inflammation and hepatic encephalopathy: ibuprofen restores learning ability in rats with portacaval shunts. Hepatology 46, 514–519 (2007).
Brück, J. et al. Locomotor impairment and cerebrocortical oxidative stress in portal vein ligated rats in vivo. J. Hepatol. 54, 251–257 (2011).
Zemtsova, I. et al. Microglia activation in hepatic encephalopathy in rats and humans. Hepatology 54, 204–215 (2011).
Andersson, A. K. et al. Lactate contributes to ammonia-mediated astroglial dysfunction during hyperammonemia. Neurochem. Res. 34, 556–565 (2009).
Duchini, A., Govindarajan, S., Santucci, M., Zampi, G. & Hofman, F. M. Effects of tumor necrosis factor-α and interleukin-6 on fluid-phase permeability and ammonia diffusion in CNS-derived endothelial cells. J. Invest. Med. 44, 474–482 (1996).
Chastre, A., Jiang, W., Desjardins, P. & Butterworth, R. F. Ammonia and proinflammatory cytokines modify expression of genes coding for astrocytic proteins implicated in brain edema in acute liver failure. Metab. Brain Dis. 25, 17–21 (2010).
Tofteng, F. et al. Cerebral microdialysis in patients with fulminant hepatic failure. Hepatology 36, 1333–1340 (2002).
Mans, A. M., DeJoseph, M. R. & Hawkins, R. A. Metabolic abnormalities and grade of encephalopathy in acute hepatic failure. J. Neurochem. 63, 1829–1838 (1994).
Zwingmann, C., Chatauret, N., Leibfritz, D. & Butterworth, R. F. Selective increase of brain lactate synthesis in experimental acute liver failure: results of a [1H-13C] nuclear magnetic resonance study. Hepatology 37, 420–428 (2003).
Chatauret, N., Zwingmann, C., Rose, C., Leibfritz, D. & Butterworth, R. F. Effects of hypothermia on brain glucose metabolism in acute liver failure: a H/C-nuclear magnetic resonance study. Gastroenterology 125, 815–824 (2003).
Rose, C. et al. Association of reduced extracellular brain ammonia, lactate, and intracranial pressure in pigs with acute liver failure. Hepatology 46, 1883–1892 (2007).
Deutz, N. E., Chamuleau R. A., de Graaf, A. A., Bovée W. M. & de Beer, R. In vivo 31P NMR spectroscopy of the rat cerebral cortex during acute hepatic encephalopathy. NMR Biomed. 1, 101–106 (1988).
Lai, J. C. & Cooper, A. J. Brain α-ketoglutarate dehydrogenase complex: kinetic properties, regional distribution, and effects of inhibitors. J. Neurochem. 47, 1376–1386 (1986).
Ratnakumari, L. & Murthy, C. R. Response of rat cerebral glycolytic enzymes to hyperammonemic states. Neurosci. Lett. 161, 37–40 (1993).
Pomier-Layrargues, G., Spahr, L. & Butterworth, R. F. Increased manganese concentrations in pallidum of cirrhotic patients. Lancet 345, 735 (1995).
Rose, C. et al. Manganese deposition in basal ganglia structures results from both portalsystemic shunting and liver dysfunction. Gastroenterology 117, 640–644 (1999).
Spahr, L. et al. Magnetic resonance imaging and proton spectroscopic alterations correlate with parkinsonian signs in patients with cirrhosis. Gastroenterology 119, 774–781 (2000).
Burkhard, P. R., Delavelle, J., Du Pasquier, R. & Spahr, L. Chronic parkinsonism associated with cirrhosis: a distinct subset of acquired hepatocerebral degeneration. Arch. Neurol. 60, 521–528 (2003).
Butterworth R. F. Parkinsonism in cirrhosis: pathogenesis and current therapeutic options. Metab. Brain Dis. http://dx.doi.org/10.1007/s11011-012-9341-7.
Dodd, C. A. & Filipov, N. M. Manganese potentiates LPS-induced heme-oxygenase 1 in microglia but not dopaminergic cells: role in controlling microglial hydrogen peroxide and inflammatory cytokine output. Neurotoxicology 32, 683–692 (2011).
Zhang, P., Lokuta, K. M., Turner, D. E. & Liu, B. Synergistic dopaminergic neurotoxicity of manganese and lipopolysaccharide: differential involvement of microglia and astroglia. J. Neurochem. 112, 434–443 (2007).
Filipov, N. M., Seegal, R. F. & Lawrence, D. A. Manganese potentiates in vitro production of proinflammatory cytokines and nitric oxide by microglia through a nuclear factor κB-dependent mechanism. Toxicol. Sci. 84, 139–148 (2005).
de Vries, H. E. et al. The influence of cytokines on the integrity of the blood–brain barrier in vitro. J. Neuroimmunol. 64, 37–43 (1996).
Didier, N. et al. Secretion of interleukin-1β by astrocytes mediates endothelin-1 and tumour necrosis factor-α effects on human brain microvascular endothelial cell permeability. J. Neurochem. 86, 246–254 (2003).
Blamire, A. M. et al. Interleukin-1β -induced changes in blood–brain barrier permeability, apparent diffusion coefficient, and cerebral blood volume in the rat brain: a magnetic resonance study. J. Neurosci. 20, 8153–8159 (2000).
Dixit, V. & Chang, T. M. Brain edema and the blood brain barrier in galactosamine-induced fulminant hepatic failure rats. An animal model for evaluation of liver support systems. ASAIO Trans. 36, 21–27 (1990).
Potvin, M., Finlayson, M. H., Hinchey, E. J., Lough, J. O. & Goresky, C. A. Cerebral abnormalities in hepatectomized rats with acute hepatic coma. Lab. Invest. 50, 560–564 (1984).
Traber, P. G., Dal Canto, M., Ganger, D. R. & Blei, A. T. Electron microscopic evaluation of brain edema in rabbits with galactosamine-induced fulminant hepatic failure: ultrastructure and integrity of the blood–brain barrier. Hepatology 7, 1272–1277 (1987).
Kato, M., Hughes, R. D., Keays, R. T. & Williams, R. Electron microscopic study of brain capillaries in cerebral edema from fulminant hepatic failure. Hepatology 15, 1060–1066 (1992).
Bélanger, M., Côté, J. & Butterworth, R. F. Neurobiological characterization of an azoxymethane mouse model of acute liver failure. Neurochem. Int. 48, 434–440 (2006).
Sawara, K. et al. Alterations in expression of genes coding for proteins of the neurovascular unit in ischemic liver failure. Neurochem. Int. 55, 119–123 (2009).
Palenzuela, L., Mas, A., Montaner, J. & Cordoba, J. Matrix metalloproteinase-9 in fulminant hepatic failure. Hepatology 51, 1475–1476 (2010).
Bémeur, C., Chastre, A., Desjardins, P. & Butterworth, R. F. No changes in expression of tight junction proteins or blood–brain barrier permeability in azoxymethane-induced experimental acute liver failure. Neurochem. Int. 56, 205–207 (2010).
Chen, F. et al. Occludin is regulated by epidermal growth factor receptor activation in brain endothelial cells and brains of mice with acute liver failure. Hepatology 53, 1294–1305 (2011).
Shimojima, N. et al. Altered expression of zonula occludens-2 precedes increased blood–brain barrier permeability in a murine model of fulminant hepatic failure. J. Invest. Surg. 21, 101–108 (2008).
Nguyen, J. H. Subtle BBB alterations in brain edema associated with acute liver failure. Neurochem. Int. 56, 203–204 (2010).
Henry, C. J., Huang, Y., Wynne, A. M. & Godbout, J. P. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1β and anti-inflammatory IL-10 cytokines. Brain Behav. Immun. 23, 309–317 (2009).
Tanaka, S. et al. Lipopolysaccharide-induced microglial activation induces learning and memory deficits without neuronal cell death in rats. J. Neurosci. Res. 83, 557–566 (2006).
Chastre, A., Bélanger, M. & Butterworth, R. F. Modest systemic inflammation precipitates encephalopathy and ruptures the blood–brain barrier in acute liver failure. Hepatology 56 (Suppl.1), 956A (2012).
Lockwood, A. H., Yap, E. W. & Wong, W. H. Cerebral ammonia metabolism in patients with severe liver disease and minimal hepatic encephalopathy. J. Cereb. Blood Flow Metab. 11, 337–341 (1991).
Ruprecht, R. et al. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric conditions. Nat. Rev. Drug Disc. 9, 971–988 (2010).
Itzhak, Y., Roig-Cantisano, A., Dombro, R. S. & Norenberg, M. D. Acute liver failure and hyperammonemia increase peripheral-type benzodiazepine receptor binding and pregnenolone synthesis in mouse brain. Brain Res. 705, 345–348 (1995).
Bélanger, M., Ahboucha, S., Desjardins, P. & Butterworth, R. F. Upregulation of peripheral-type (mitochondrial) benzodiazepine receptors in hyperammonemic syndromes: consequences for neuronal excitability. Adv. Mol. Cell Biol. 31, 983–997 (2004).
Giguère, J. F., Hamel, E. & Butterworth, R. F. Increased densities of binding sites for the 'peripheral-type' benzodiazepine receptor ligand [3H]PK 11195 in rat brain following portacaval anastomosis. Brain Res. 585, 295–298 (1992).
Desjardins, P., Bandeira, P., Rao, V. L. & Butterworth, R. F. Portacaval anastomosis causes selective alterations of peripheral-type benzodiazepine receptor expression in rat brain and peripheral tissues. Neurochem. Int. 35, 293–299 (1999).
Lavoie, J., Layrargues, G. P. & Butterworth, R. F. Increased densities of peripheral-type benzodiazepine receptors in brain autopsy samples from cirrhotic patients with hepatic encephalopathy. Hepatology 11, 874–878 (1990).
Oh, U. et al. Translocator protein PET imaging for glial activation in multiple sclerosis. J. Neuroimmune Pharmacol. 6, 354–361 (2011).
Cagnin, A., Taylor-Robinson, S. D., Forton, D. M. & Banati R. B. In vivo imaging of cerebral “peripheral benzodiazepine binding sites” in patients with hepatic encephalopathy. Gut 55, 547–553 (2006).
Prasad, S. et al. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology 45, 549–559 (2007).
Bass, N. M. et al. Rifaximin treatment in hepatic encephalopathy. N. Engl. J. Med. 362, 1071–1081 (2010).
Kircheis, G. et al. Therapeutic efficacy of L-ornithine-L-aspartate infusions in patients with cirrhosis and hepatic encephalopathy: results of a placebo-controlled, double-blind study. Hepatology 25, 1351–1360 (1997).
Odeh, M., Sabo, E., Srugo, I. & Oliven, A. Serum levels of tumor necrosis factor-α correlate with severity of hepatic encephalopathy due to chronic liver failure. Liver Int. 24, 110–116 (2004).
Wright, G. et al. Reduction in hyperammonemia by ornithine phenylacetate prevents lipopolysaccharide-induced brain edema and coma in cirrhotic rats. Liver Int. 32, 410–419 (2012).
Agrawal, A., Sharma, B. C., Sharma, P. & Sarin, S. K. Secondary prophylaxis of hepatic encephalopathy in cirrhosis: an open-label, randomized controlled trial of lactulose, probiotics, and no therapy. Am. J. Gastroenterol. 107, 1043–1050 (2012).
Cauli, O. et al. Neuroinflammation contributes to hypokinesia in rats with hepatic encephalopathy: ibuprofen restores its motor activity. J. Neurosci. Res. 87, 1369–1374 (2009).
Chastre, A. et al. Inflammatory cascades driven by tumor necrosis factor-α play a major role in the progression of acute liver failure and its neurological complications. PLoS ONE 7, e49670 (2012).
Guo, L. M. et al. Application of Molecular Adsorbents Recirculating System to remove NO and cytokines in severe liver failure patients with multiple organ dysfunction syndrome. Liver Int. 23 (Suppl.3), 16–20 (2003).
Jalan, R., Olde Daminck, S. W., Deutz, M. E., Lee, A. & Hayes, P. C. Moderate hypothermia for uncontrolled intracranial hypertension in acute liver failure. Lancet 354, 1164–1168 (1999).
Vaquero, J. et al. Mild hypothermia attenuates liver injury and improves survival in mice with acetaminophen toxicity. Gastroenterology 132, 372–383 (2007).
Jiang, W., Desjardins, P. & Butterworth, R. F. Direct evidence for central proinflammatory mechanisms in rats with experimental acute liver failure: protective effect of hypothermia. J. Cereb. Blood Flow Metab. 29, 944–952 (2009).
Chen, G., Shi, J., Hu, Z. & Hang, C. Inhibitory effect on cerebral inflammatory response following traumatic brain injury in rats: a potential neuroprotective mechanism of N-acetylcysteine. Mediators Inflamm. 2008, 716458 (2008).
Stirling, D. P., Koochesfahani, K. M., Steeves, J. D. & Tetzlaff, W. Minocycline as a neuroprotective agent. Neuroscientist 11, 308–322 (2005).
Jiang, W., Desjardins, P. & Butterworth, R. F. Minocycline attenuates oxidative/nitrosative stress and cerebral complications of acute liver failure in rats. Neurochem. Int. 55, 601–605 (2009).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
R. F. Butterworth has served as consultant and benefited from financial support as a speaker at conferences in an ad hoc manner from the following pharmaceutical companies: Merz Pharmaceuticals, Otsuka Pharmaceuticals and Salix Pharmaceuticals. The author and his family have no direct financial interests in any of these companies.
Rights and permissions
About this article
Cite this article
Butterworth, R. The liver–brain axis in liver failure: neuroinflammation and encephalopathy. Nat Rev Gastroenterol Hepatol 10, 522–528 (2013). https://doi.org/10.1038/nrgastro.2013.99
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2013.99
This article is cited by
-
Prophylaxis of hepatic encephalopathy: current and future drug targets
Hepatology International (2024)
-
Regulatory Effects and Mechanisms of L-Theanine on Neurotransmitters via Liver–Brain Axis Under a High Protein Diet
Molecular Neurobiology (2024)
-
Circadian Regulation of the Lactate Metabolic Kinetics in Mice Using the [1H-13C]-NMR Technique
Molecular Neurobiology (2024)
-
Circular RNA Tmcc1 improves astrocytic glutamate metabolism and spatial memory via NF-κB and CREB signaling in a bile duct ligation mouse model: transcriptional and cellular analyses
Journal of Neuroinflammation (2023)
-
The brain-liver cholinergic anti-inflammatory pathway and viral infections
Bioelectronic Medicine (2023)