Abstract
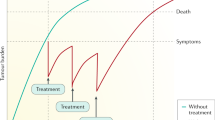
The antiangiogenic multikinase inhibitor sorafenib was the first systemic agent to demonstrate a significant improvement in the overall survival of patients with advanced hepatocellular carcinoma (HCC), thereby introducing molecularly-targeted therapy in a therapeutic field of unmet needs. However, survival benefits for patients on sorafenib treatment are modest in clinical practice and advancing the field is far more challenging than initially anticipated. Molecular and clinical heterogeneity diminishes signals of potential activity in unselected populations, and underlying liver cirrhosis seals the fate of many novel targeted agents by causing relevant toxicity and mortality. The failure of subsequent randomized controlled phase III trials underscores the urgent need to identify the driver targets and to develop matched active agents with manageable toxicities in specific phase I studies in patients with cirrhosis. Refinement of phase II–III trial designs with a biomarker-enriched patient-selection process and stratification according to prognostic baseline factors is indispensable to prevent another 5-year vain endeavour in systemic therapy of HCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Jemal, A. et al. Global cancer statistics. CA Cancer J. Clin. 61, 69–90 (2011).
Worns, M. A., Weinmann, A., Schuchmann, M. & Galle, P. R. Systemic therapies in hepatocellular carcinoma. Dig. Dis. 27, 175–188 (2009).
Llovet, J. M. et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390 (2008).
Cheng, A. L. et al. Efficacy and safety of sorafenib in patients in the Asia–Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 10, 25–34 (2009).
Bruix, J. & Sherman, M. Management of hepatocellular carcinoma: an update. Hepatology 53, 1020–1022 (2011).
EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 56, 908–943 (2012).
Worns, M. A. & Galle, P. R. Novel inhibitors in development for hepatocellular carcinoma. Expert Opin. Investig. Drugs 19, 615–629 (2010).
Llovet, J. M. et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J. Natl Cancer Inst. 100, 698–711 (2008).
Worns, M. A. Systemic therapy and synergies by combination. Dig. Dis. 31, 104–111 (2013).
Cheng, A. L. et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J. Clin. Oncol. 31, 4067–4075 (2013).
Cainap, C. et al. Phase III trial of linifanib versus sorafenib in patients with advanced hepatocellular carcinoma (HCC) [abstract]. J. Clin. Oncol. 30 (Suppl. 34), 249 (2013).
Johnson, P. J. et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J. Clin. Oncol. 31, 3517–3524 (2013).
Lencioni, R. & Llovet, J. M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 30, 52–60 (2010).
Zhu, A. X. et al. SEARCH: A phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma (HCC) [abstract]. Eur. J. Cancer 48 (Suppl.) 2 (2012).
Qin, S. et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J. Clin. Oncol. 31, 3501–3508 (2013).
Llovet, J. M. et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J. Clin. Oncol. 31, 3509–3516 (2013).
Novartis study of Afinitor® in advanced liver cancer does not meet primary endpoint of overall survival. Novartis [online], (2013).
Worns, M. A. & Galle, P. R. Future perspectives in hepatocellular carcinoma. Dig. Liver Dis. 42 (Suppl. 3), S302–S309 (2010).
Villanueva, A. & Llovet, J. M. Second-line therapies in hepatocellular carcinoma: emergence of resistance to sorafenib. Clin. Cancer Res. 18, 1824–1826 (2012).
Villanueva, A. Rethinking future development of molecular therapies in hepatocellular carcinoma: a bottom-up approach. J. Hepatol. 59, 392–395 (2013).
Kelley, R. K. & Venook, A. P. Novel therapeutics in hepatocellular carcinoma. Am. Soc. Clin. Oncol. Educ. Book 2013, 137–142 (2013).
Llovet, J. M., Bru, C. & Bruix, J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin. Liver Dis. 19, 329–338 (1999).
Forner, A., Llovet, J. M. & Bruix, J. Hepatocellular carcinoma. Lancet 379, 1245–1255 (2012).
Villanueva, A., Newell, P., Chiang, D. Y., Friedman, S. L. & Llovet, J. M. Genomics and signaling pathways in hepatocellular carcinoma. Semin. Liver Dis. 27, 55–76 (2007).
Weinstein, I. B. & Joe, A. Oncogene addiction. Cancer Res. 68, 3077–3080 (2008).
Shaw, A. T. et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N. Engl. J. Med. 368, 2385–2394 (2013).
Chapman, P. B. et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 (2011).
Marquardt, J. U. & Andersen, J. B. Next-generation sequencing: application in liver cancer—past, present and future? Biology (Basel) 1, 383–394 (2012).
Schirmacher, P. et al. Fighting the bushfire in HCC trials. J. Hepatol. 55, 276–277 (2011).
Villanueva, A. & Llovet, J. M. Impact of intra-individual molecular heterogeneity in personalized treatment of hepatocellular carcinoma. Hepatology 56, 2416–2419 (2012).
Pantel, K. & Alix-Panabieres, C. Real-time liquid biopsy in cancer patients: fact or fiction? Cancer Res. 73, 6384–6388 (2013).
Santoro, A. et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol. 14, 55–63 (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Schmieder, R. et al. Allosteric MEK1/2 inhibitor refametinib (BAY 86–9766) in combination with sorafenib exhibits antitumor activity in preclinical murine and rat models of hepatocellular carcinoma. Neoplasia 15, 1161–1171 (2013).
Zhu, A. X. et al. First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 19, 920–928 (2013).
Catalogue of Somatic Mutations in Cancer [online].
Assenat, E. et al. Sorafenib alone versus sorafenib combined with gemcitabine and oxaliplatin (GEMOX) in the first-line treatment of advanced hepatocellular carcinoma: final results of the randomized phase II GoNext trial (a UNICANCER/FFCD/PRODIGE 10 study) [abstract]. Eur. J. Cancer 49 (Suppl. 2), 2467 (2013).
US National Library of Medicine ClinicalTrials.gov [online], (2013).
Llovet, J. M., Burroughs, A. & Bruix, J. Hepatocellular carcinoma. Lancet 362, 1907–1917 (2003).
ArQule Announces Third Quarter Fiscal 2013 Results. Arqule [online], (2013).
Reig, M. et al. Postprogression survival of patients with advanced hepatocellular carcinoma: rationale for second line trial design. Hepatology 58, 2023–2031 (2013).
Kelley, R. K. Brivanib and FOLFOX in hepatocellular carcinoma: finding the common themes among negative trials. J. Clin. Oncol. 31, 3483–3486 (2013).
Shao, Y. Y. et al. Prognosis of patients with advanced hepatocellular carcinoma who failed first-line systemic therapy. J. Hepatol. 60, 313–318 (2014).
He, A. R. & Goldenberg, A. S. Treating hepatocellular carcinoma progression following first-line sorafenib: therapeutic options and clinical observations. Therap. Adv. Gastroenterol. 6, 447–458 (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
Author information
Authors and Affiliations
Contributions
M.-A.W. contributed to researching data, discussing the content and writing the manuscript. P.R.G. contributed to researching data, discussing the content, and reviewing/editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.-A. Wörns and P. R. Galle have received consulting and lecture fees from Bayer HealthCare and Bristol-Myers Squibb.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Wörns, MA., Galle, P. HCC therapies—lessons learned. Nat Rev Gastroenterol Hepatol 11, 447–452 (2014). https://doi.org/10.1038/nrgastro.2014.10
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2014.10
This article is cited by
-
miR-29a-SIRT1-Wnt/β-Catenin Axis Regulates Tumor Progression and Survival in Hepatocellular Carcinoma
Biochemical Genetics (2023)
-
Emerging Therapeutic Targets for Portal Hypertension
Current Hepatology Reports (2023)
-
A VEGFR targeting peptide-drug conjugate (PDC) suppresses tumor angiogenesis in a TACE model for hepatocellular carcinoma therapy
Cell Death Discovery (2022)
-
Hypoxic Hepatocellular Carcinoma Cells Acquire Arsenic Trioxide Resistance by Upregulating HIF-1α Expression
Digestive Diseases and Sciences (2022)
-
Molecular classification of hepatocellular carcinoma: prognostic importance and clinical applications
Journal of Cancer Research and Clinical Oncology (2022)