Key Points
-
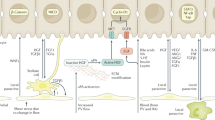
Liver regeneration occurs efficiently in the normal liver to restore architecture, size and function; chronic injury severely impairs liver regeneration through excess inflammation, scarring and epithelial abnormalities, and is less well-studied but clinically important
-
New experimental models are emerging; zebrafish are an excellent new tool to study liver regeneration and enable large-scale chemical screening assays
-
A gap exists between current animal models of liver regeneration and clinically important scenarios of severe liver injury and impaired liver regeneration
-
Understanding and promoting regeneration and repair of the failing liver is a key challenge of major clinical importance
-
Modern imaging techniques will enable noninvasive real-time assessment of liver structure and function
-
Cell therapies that have been successful in animal models are now being trialled in the more challenging clinical arena
Abstract
Liver regeneration has been studied for many decades and the mechanisms underlying regeneration of the normal liver following resection or moderate damage are well described. A large number of factors extrinsic (such as bile acids and circulating growth factors) and intrinsic to the liver interact to initiate and regulate liver regeneration. Less well understood, and more clinically relevant, are the factors at play when the abnormal liver is required to regenerate. Fatty liver disease, chronic scarring, prior chemotherapy and massive liver injury can all inhibit the normal programme of regeneration and can lead to liver failure. Understanding these mechanisms could enable the rational targeting of specific therapies to either reduce the factors inhibiting regeneration or directly stimulate liver regeneration. Although animal models of liver regeneration have been highly instructive, the clinical relevance of some models could be improved to bridge the gap between our in vivo model systems and the clinical situation. Likewise, modern imaging techniques such as spectroscopy will probably improve our understanding of whole-organ metabolism and how this predicts the liver's regenerative capacity. This Review describes briefly the mechanisms underpinning liver regeneration, the models used to study this process, and discusses areas in which failed or compromised liver regeneration is clinically relevant.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Issa, R. et al. Mutation in collagen-1 that confers resistance to the action of collagenase results in failure of recovery from CCl4-induced liver fibrosis, persistence of activated hepatic stellate cells, and diminished hepatocyte regeneration. FASEB J. 17, 47–49 (2003).
Bhushan, B. et al. Pro-regenerative signaling after acetaminophen-induced acute liver injury in mice identified using a novel incremental dose model. Am. J. Pathol. 184, 3013–3025 (2014).
Bernal, W., Lee, W. M., Wendon, J., Larsen, F. S. & Williams, R. Acute liver failure: a curable disease by 2024? J. Hepatol. 62, S112–S120 (2015).
Michalopoulos, G. K. & DeFrances, M. C. Liver regeneration. Science 276, 60–66 (1997).
Bird, T. G., Lorenzini, S. & Forbes, S. J. Activation of stem cells in hepatic diseases. Cell Tissue Res. 331, 283–300 (2008).
Lauterio, A. et al. Current status and perspectives in split liver transplantation. World J. Gastroenterol. 21, 11003–11015 (2015).
Eshkenazy, R. et al. Small for size liver remnant following resection: prevention and management. Hepatobiliary Surg. Nutr. 3, 303–312 (2014).
Ploeg, R. J. et al. Risk factors for primary dysfunction after liver transplantation — a multivariate analysis. Transplantation 55, 807–813 (1993).
Asrani, S. K. & Kamath, P. S. Natural history of cirrhosis. Curr. Gastroenterol. Rep. 15, 308 (2013).
Forbes, S. J. et al. Retroviral gene transfer to the liver in vivo during tri-iodothyronine induced hyperplasia. Gene Ther. 5, 552–555 (1998).
Higgins, G. M. & Anderson, R. M. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch. Pathol. Lab. Med. 12, 186–202 (1931).
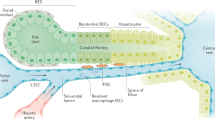
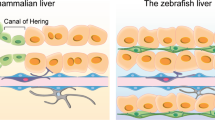
He, J., Lu, H., Zou, Q. & Luo, L. Regeneration of liver after extreme hepatocyte loss occurs mainly via biliary transdifferentiation in zebrafish. Gastroenterology 146, 789–800.e8 (2014).
Forbes, S. J. & Rosenthal, N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat. Med. 20, 857–869 (2014).
Moolten, F. L. & Bucher, N. L. Regeneration of rat liver: transfer of humoral agent by cross circulation. Science 158, 272–274 (1967).
Nakamura, R. M., Miyada, D. S. & Moyer, D. L. Effect of liver regeneration following partial hepatectomy on the uptake of tritiated thymidine in the pituitary gland of the rat. Nature 199, 707–708 (1963).
Michalopoulos, G. K. Liver regeneration. J. Cell. Physiol. 213, 286–300 (2007).
Martins, P. N., Theruvath, T. P. & Neuhaus, P. Rodent models of partial hepatectomies. Liver Int. 28, 3–11 (2008).
Demetris, A. J. et al. Pathophysiologic observations and histopathologic recognition of the portal hyperperfusion or small-for-size syndrome. Am. J. Surg. Pathol. 30, 986–993 (2006).
Ren, W. et al. Selective bowel decontamination improves the survival of 90% hepatectomy in rats. J. Surg. Res. 195, 454–464 (2015).
Capussotti, L. et al. Liver dysfunction and sepsis determine operative mortality after liver resection. Br. J. Surg. 96, 88–94 (2009).
Busani, S. et al. Living donor liver transplantation and management of portal venous pressure. Transplant. Proc. 38, 1074–1075 (2006).
Du, Z. et al. Octreotide prevents liver failure through upregulating 5′-methylthioadenosine in extended hepatectomized rats. Liver Int. 36, 212–222 (2016).
Ninomiya, M. et al. Deceleration of regenerative response improves the outcome of rat with massive hepatectomy. Am. J. Transplant. 10, 1580–1587 (2010).
Fujio, K., Evarts, R. P., Hu, Z., Marsden, E. R. & Thorgeirsson, S. S. Expression of stem cell factor and its receptor, c-kit, during liver regeneration from putative stem cells in adult rat. Lab. Invest. 70, 511–516 (1994).
Ghoshal, A. K., Mullen, B., Medline, A. & Farber, E. Sequential analysis of hepatic carcinogenesis. Regeneration of liver after carbon tetrachloride-induced liver necrosis when hepatocyte proliferation is inhibited by 2-acetylaminofluorene. Lab. Invest. 48, 224–230 (1983).
Evarts, R. P., Nagy, P., Marsden, E. & Thorgeirsson, S. S. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis 8, 1737–1740 (1987).
Trautwein, C. et al. 2-acetaminofluorene blocks cell cycle progression after hepatectomy by p21 induction and lack of cyclin E expression. Oncogene 18, 6443–6453 (1999).
Dusabineza, A. C. et al. Participation of liver progenitor cells in liver regeneration: lack of evidence in the AAF/PH rat model. Lab. Invest. 92, 72–81 (2012).
Lemaigre, F. P. Determining the fate of hepatic cells by lineage tracing: facts and pitfalls. Hepatology 61, 2100–2103 (2015).
Bockamp, E. et al. Conditional transgenic mouse models: from the basics to genome-wide sets of knockouts and current studies of tissue regeneration. Regen. Med. 3, 217–235 (2008).
Nikfarjam, M., Malcontenti-Wilson, C., Fanartzis, M., Daruwalla, J. & Christophi, C. A model of partial hepatectomy in mice. J. Invest. Surg. 17, 291–294 (2004).
Iredale, J. P. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J. Clin. Invest. 117, 539–548 (2007).
Duffield, J. S. et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest. 115, 56–65 (2005).
Ramachandran, P. et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc. Natl Acad. Sci. USA 109, E3186–E3195 (2012).
Kallis, Y. N. et al. Remodelling of extracellular matrix is a requirement for the hepatic progenitor cell response. Gut 60, 525–533 (2011).
Preisegger, K. H. et al. Atypical ductular proliferation and its inhibition by transforming growth factor beta1 in the 3,5-diethoxycarbonyl-1,4-dihydrocollidine mouse model for chronic alcoholic liver disease. Lab. Invest. 79, 103–109 (1999).
Hsieh, W. C. et al. Galectin-3 regulates hepatic progenitor cell expansion during liver injury. Gut 64, 312–321 (2015).
Williams, M. J., Clouston, A. D. & Forbes, S. J. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology 146, 349–356 (2014).
Akhurst, B. et al. A modified choline-deficient, ethionine-supplemented diet protocol effectively induces oval cells in mouse liver. Hepatology 34, 519–522 (2001).
Boulter, L. et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat. Med. 18, 572–579 (2012).
Tsuchiya, A. et al. Polysialic acid/neural cell adhesion molecule modulates the formation of ductular reactions in liver injury. Hepatology 60, 1727–1740 (2014).
Kim, K. H., Chen, C. C., Alpini, G. & Lau, L. F. CCN1 induces hepatic ductular reaction through integrin αvβ5-mediated activation of NF-κB. J. Clin. Invest. 125, 1886–1900 (2015).
Yanger, K. et al. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell Stem Cell 15, 340–349 (2014).
Jors, S. et al. Lineage fate of ductular reactions in liver injury and carcinogenesis. J. Clin. Invest. 125, 2445–2457 (2015).
Lu, W. Y. et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat. Cell Biol. 17, 971–983 (2015).
Cox, A. G. & Goessling, W. The lure of zebrafish in liver research: regulation of hepatic growth in development and regeneration. Curr. Opin. Genet. Dev. 32, 153–161 (2015).
Curado, S., Stainier, D. Y. & Anderson, R. M. Nitroreductase-mediated cell/tissue ablation in zebrafish: a spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nat. Protoc. 3, 948–954 (2008).
Sadler, K. C., Krahn, K. N., Gaur, N. A. & Ukomadu, C. Liver growth in the embryo and during liver regeneration in zebrafish requires the cell cycle regulator, uhrf1. Proc. Natl Acad. Sci. USA 104, 1570–1575 (2007).
Goessling, W. et al. APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development. Dev. Biol. 320, 161–174 (2008).
Vliegenthart, A. D., Tucker, C. S., Del Pozo, J. & Dear, J. W. Zebrafish as model organisms for studying drug-induced liver injury. Br. J. Clin. Pharmacol. 78, 1217–1227 (2014).
Choi, T. Y., Ninov, N., Stainier, D. Y. & Shin, D. Extensive conversion of hepatic biliary epithelial cells to hepatocytes after near total loss of hepatocytes in zebrafish. Gastroenterology 146, 776–788 (2014).
Verfaillie, C. M. Biliary cells to the rescue of Prometheus. Gastroenterology 146, 611–614 (2014).
Huang, M. et al. Antagonistic interaction between Wnt and Notch activity modulates the regenerative capacity of a zebrafish fibrotic liver model. Hepatology 60, 1753–1766 (2014).
Jiang, F. et al. Analysis of mutants from a genetic screening reveals the control of intestine and liver development by many common genes in zebrafish. Biochem. Biophys. Res. Commun. 460, 838–844 (2015).
Schaub, J. R., Malato, Y., Gormond, C. & Willenbring, H. Evidence against a stem cell origin of new hepatocytes in a common mouse model of chronic liver injury. Cell Rep. 8, 933–939 (2014).
Tarlow, B. D. et al. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell 15, 605–618 (2014).
Yanger, K. et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 27, 719–724 (2013).
Stueck, A. E. & Wanless, I. R. Hepatocyte buds derived from progenitor cells repopulate regions of parenchymal extinction in human cirrhosis. Hepatology 61, 1696–1707 (2015).
Lin, W. R. et al. The histogenesis of regenerative nodules in human liver cirrhosis. Hepatology 51, 1017–1026 (2010).
Ueda, J., Chijiiwa, K., Nakano, K., Zhao, G. & Tanaka, M. Lack of intestinal bile results in delayed liver regeneration of normal rat liver after hepatectomy accompanied by impaired cyclin E-associated kinase activity. Surgery 131, 564–573 (2002).
Huang, W. et al. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science 312, 233–236 (2006).
Meng, Z. et al. FXR regulates liver repair after CCl4-induced toxic injury. Mol. Endocrinol. 24, 886–897 (2010).
Otao, R. et al. External biliary drainage and liver regeneration after major hepatectomy. Br. J. Surg. 99, 1569–1574 (2012).
Kele, P. G., de Boer, M., van der Jagt, E. J., Lisman, T. & Porte, R. J. Early hepatic regeneration index and completeness of regeneration at 6 months after partial hepatectomy. Br. J. Surg. 99, 1113–1119 (2012).
Black, S. M., Whitson, B. A. & Velayutham, M. EPR spectroscopy as a predictive tool for the assessment of marginal donor livers perfused on a normothermic ex vivo perfusion circuit. Med. Hypotheses 82, 627–630 (2014).
Qi, J. et al. 31P MR spectroscopic imaging detects regenerative changes in human liver stimulated by portal vein embolization. J. Magn. Reson. Imaging 34, 336–344 (2011).
Zakian, K. L. et al. Liver regeneration in humans is characterized by significant changes in cellular phosphorus metabolism: assessment using proton-decoupled 31P-magnetic resonance spectroscopic imaging. Magn. Reson. Med. 54, 264–271 (2005).
Kumar, S., Zou, Y., Bao, Q., Wang, M. & Dai, G. Proteomic analysis of immediate-early response plasma proteins after 70% and 90% partial hepatectomy. Hepatol. Res. 43, 876–889 (2013).
Afolabi, P., Wright, M., Wootton, S. A. & Jackson, A. A. Clinical utility of 13C-liver-function breath tests for assessment of hepatic function. Dig. Dis. Sci. 58, 33–41 (2013).
Miura, Y., Washizawa, N., Urita, Y., Imai, T. & Kaneko, H. Evaluation of remnant liver function using 13C-breath tests in a rat model of 70% partial hepatectomy. Hepatogastroenterology 59, 311–316 (2012).
Lee, S. G. A complete treatment of adult living donor liver transplantation: a review of surgical technique and current challenges to expand indication of patients. Am. J. Transplant. 15, 17–38 (2015).
Apte, U. et al. Beta-catenin activation promotes liver regeneration after acetaminophen-induced injury. Am. J. Pathol. 175, 1056–1065 (2009).
Holt, M. P., Cheng, L. & Ju, C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J. Leukoc. Biol. 84, 1410–1421 (2008).
Amemiya, H., Kono, H. & Fujii, H. Liver regeneration is impaired in macrophage colony stimulating factor deficient mice after partial hepatectomy: the role of M-CSF-induced macrophages. J. Surg. Res. 165, 59–67 (2011).
Stutchfield, B. M. et al. CSF1 restores innate immunity after liver injury in mice and serum levels indicate outcomes of patients with acute liver failure. Gastroenterology 149, 1896–1909.e14 (2015).
Vetelainen, R., van Vliet, A. K. & van Gulik, T. M. Severe steatosis increases hepatocellular injury and impairs liver regeneration in a rat model of partial hepatectomy. Ann. Surg. 245, 44–50 (2007).
Truant, S. et al. Volumetric gain of the liver after major hepatectomy in obese patients: a case-matched study in 84 patients. Ann. Surg. 258, 696–702; discussion 702–704 (2013).
Younossi, Z. M. et al. Global epidemiology of non-alcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence and outcomes. Hepatology http://dx.doi.org/10.1002/hep.28431 (2015).
Verran, D. et al. Clinical experience gained from the use of 120 steatotic donor livers for orthotopic liver transplantation. Liver Transpl. 9, 500–505 (2003).
Chu, M. J., Dare, A. J., Phillips, A. R. & Bartlett, A. S. Donor hepatic steatosis and outcome after liver transplantation: a systematic review. J. Gastrointest. Surg. 19, 1713–1724 (2015).
Rogier, J. et al. Noninvasive assessment of macrovesicular liver steatosis in cadaveric donors based on computed tomography liver-to-spleen attenuation ratio. Liver Transpl. 21, 690–695 (2015).
Hewitt, K. C. et al. Accurate assessment of liver steatosis in animal models using a high throughput Raman fiber optic probe. Analyst 140, 6602–6609 (2015).
Inaba, Y. et al. Growth arrest and DNA damage-inducible 34 regulates liver regeneration in hepatic steatosis in mice. Hepatology 61, 1343–1356 (2015).
Shimamura, T. et al. Excessive portal venous inflow as a cause of allograft dysfunction in small-for-size living donor liver transplantation. Transplant. Proc. 33, 1331 (2001).
Dahm, F., Georgiev, P. & Clavien, P. A. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am. J. Transplant. 5, 2605–2610 (2005).
de Rougemont, O., Lehmann, K. & Clavien, P. A. Preconditioning, organ preservation, and postconditioning to prevent ischemia-reperfusion injury to the liver. Liver Transpl. 15, 1172–1182 (2009).
Gracia-Sancho, J., Casillas-Ramirez, A. & Peralta, C. Molecular pathways in protecting the liver from ischaemia/reperfusion injury: a 2015 update. Clin. Sci. (Lond.) 129, 345–362 (2015).
Zeng, S. et al. Blockade of receptor for advanced glycation end product (RAGE) attenuates ischemia and reperfusion injury to the liver in mice. Hepatology 39, 422–432 (2004).
Cataldegirmen, G. et al. RAGE limits regeneration after massive liver injury by coordinated suppression of TNF-α and NF-κB. J. Exp. Med. 201, 473–484 (2005).
Basta, G., Del Turco, S., Navarra, T., Lee, W. M. & The Acute Liver Failure Study Group. Circulating levels of soluble receptor for advanced glycation end products and ligands of the receptor for advanced glycation end products in patients with acute liver failure. Liver Transpl. 21, 847–854 (2015).
Koh, E. J., Yoon, S. J. & Lee, S. M. Losartan protects liver against ischaemia/reperfusion injury through PPAR-γ activation and receptor for advanced glycation end-products down-regulation. Br. J. Pharmacol. 169, 1404–1416 (2013).
Henderson, N. C. & Forbes, S. J. Hepatic fibrogenesis: from within and outwith. Toxicology 254, 130–135 (2008).
Pellicoro, A. et al. Elastin accumulation is regulated at the level of degradation by macrophage metalloelastase (MMP-12) during experimental liver fibrosis. Hepatology 55, 1965–1975 (2012).
Marshall, A. et al. Relation between hepatocyte G1 arrest, impaired hepatic regeneration, and fibrosis in chronic hepatitis C virus infection. Gastroenterology 128, 33–42 (2005).
Bird, T. G. et al. Bone marrow injection stimulates hepatic ductular reactions in the absence of injury via macrophage-mediated TWEAK signaling. Proc. Natl Acad. Sci. USA 110, 6542–6547 (2013).
Thomas, J. A. et al. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology 53, 2003–2015 (2011).
D'Ambrosio, R. et al. A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis. Hepatology 56, 532–543 (2012).
Mallet, V. et al. Brief communication: the relationship of regression of cirrhosis to outcome in chronic hepatitis C. Ann. Intern. Med. 149, 399–403 (2008).
Bruix, J. et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 111, 1018–1022 (1996).
Santambrogio, R. et al. Hepatic resection for hepatocellular carcinoma in patients with Child-Pugh's A cirrhosis: is clinical evidence of portal hypertension a contraindication? HPB (Oxford) 15, 78–84 (2013).
Mazzaferro, V. et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 334, 693–699 (1996).
Tandon, P. et al. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl. 18, 1209–1216 (2012).
Weissenborn, K., Ruckert, N., Hecker, H. & Manns, M. P. The number connection tests A and B: interindividual variability and use for the assessment of early hepatic encephalopathy. J. Hepatol. 28, 646–653 (1998).
Younossi, Z. M., Guyatt, G., Kiwi, M., Boparai, N. & King, D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut 45, 295–300 (1999).
Pugh, R. N., Murray-Lyon, I. M., Dawson, J. L., Pietroni, M. C. & Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 60, 646–649 (1973).
Angermayr, B. et al. Child-Pugh versus MELD score in predicting survival in patients undergoing transjugular intrahepatic portosystemic shunt. Gut 52, 879–885 (2003).
Schiodt, F. V. et al. Alpha-fetoprotein and prognosis in acute liver failure. Liver Transpl. 12, 1776–1781 (2006).
John, K. et al. MicroRNAs play a role in spontaneous recovery from acute liver failure. Hepatology 60, 1346–1355 (2014).
Rutherford, A. et al. Development of an accurate index for predicting outcomes of patients with acute liver failure. Gastroenterology 143, 1237–1243 (2012).
Kamath, P. S., Kim, W. R. & The Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD). Hepatology 45, 797–805 (2007).
Malinchoc, M. et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 31, 864–871 (2000).
Merion, R. M. et al. Longitudinal assessment of mortality risk among candidates for liver transplantation. Liver Transpl. 9, 12–18 (2003).
Watkins, P. B. et al. Erythromycin breath test as an assay of glucocorticoid-inducible liver cytochromes P-450. Studies in rats and patients. J. Clin. Invest. 83, 688–697 (1989).
Wahllander, A., Mohr, S. & Paumgartner, G. Assessment of hepatic function. Comparison of caffeine clearance in serum and saliva during the day and at night. J. Hepatol. 10, 129–137 (1990).
Keiding, S. Galactose clearance measurements and liver blood flow. Gastroenterology 94, 477–481 (1988).
Everson, G. T. et al. Portal-systemic shunting in patients with fibrosis or cirrhosis due to chronic hepatitis C: the minimal model for measuring cholate clearances and shunt. Aliment. Pharmacol. Ther. 26, 401–410 (2007).
Cassinotto, C. et al. Non-invasive assessment of liver fibrosis with impulse elastography: comparison of Supersonic Shear Imaging with ARFI and FibroScan®. J. Hepatol. 61, 550–557 (2014).
Pandharipande, P. V., Krinsky, G. A., Rusinek, H. & Lee, V. S. Perfusion imaging of the liver: current challenges and future goals. Radiology 234, 661–673 (2005).
Regini, F. et al. Assessment of liver perfusion by intravoxel incoherent motion (IVIM) magnetic resonance-diffusion-weighted imaging: correlation with phase-contrast portal venous flow measurements. J. Comput. Assist. Tomogr. 39, 365–372 (2015).
Shikare, S. V., Bashir, K., Abraham, P. & Tilve, G. H. Hepatic perfusion index in portal hypertension of cirrhotic and non-cirrhotic aetiologies. Nucl. Med. Commun. 17, 520–522 (1996).
Marcellin, P. et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N. Engl. J. Med. 359, 2442–2455 (2008).
Pearlman, B. L., Ehleben, C. & Perrys, M. The combination of simeprevir and sofosbuvir is more effective than that of peginterferon, ribavirin, and sofosbuvir for patients with hepatitis C-related child's class A cirrhosis. Gastroenterology 148, 762–770.e2 (2014).
Dowman, J. K., Armstrong, M. J., Tomlinson, J. W. & Newsome, P. N. Current therapeutic strategies in non-alcoholic fatty liver disease. Diabetes Obes. Metab. 13, 692–702 (2011).
Dyson, J. K. et al. Unmet clinical need in autoimmune liver diseases. J. Hepatol. 62, 208–218 (2015).
Eksteen, B., Afford, S. C., Wigmore, S. J., Holt, A. P. & Adams, D. H. Immune-mediated liver injury. Semin. Liver Dis. 27, 351–366 (2007).
Yannaki, E. et al. G-CSF-primed hematopoietic stem cells or G-CSF per se accelerate recovery and improve survival after liver injury, predominantly by promoting endogenous repair programs. Exp. Hematol. 33, 108–119 (2005).
Piscaglia, A. C., Shupe, T. D., Oh, S. H., Gasbarrini, A. & Petersen, B. E. Granulocyte-colony stimulating factor promotes liver repair and induces oval cell migration and proliferation in rats. Gastroenterology 133, 619–631 (2007).
Garg, V. et al. Granulocyte colony-stimulating factor mobilizes CD34+ cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology 142, 505–512.e1 (2012).
Fanti, M., Singh, S., Ledda-Columbano, G. M., Columbano, A. & Monga, S. P. Tri-iodothyronine induces hepatocyte proliferation by protein kinase A-dependent β-catenin activation in rodents. Hepatology 59, 2309–2320 (2014).
Perra, A. et al. Thyroid hormone (T3) and TRβ agonist GC-1 inhibit/reverse nonalcoholic fatty liver in rats. FASEB J. 22, 2981–2989 (2008).
Perra, A., Kowalik, M. A., Pibiri, M., Ledda-Columbano, G. M. & Columbano, A. Thyroid hormone receptor ligands induce regression of rat preneoplastic liver lesions causing their reversion to a differentiated phenotype. Hepatology 49, 1287–1296 (2009).
Malik, R., Habib, M., Tootle, R. & Hodgson, H. Exogenous thyroid hormone induces liver enlargement, whilst maintaining regenerative potential — a study relevant to donor preconditioning. Am. J. Transplant. 5, 1801–1807 (2005).
Mu, X. et al. Hepatocellular carcinoma originates from hepatocytes and not from the progenitor/biliary compartment. J. Clin. Invest. 125, 3891–3903 (2015).
Schuppan, D. & Kim, Y. O. Evolving therapies for liver fibrosis. J. Clin. Invest. 123, 1887–1901 (2013).
Rambaldi, A. & Gluud, C. Colchicine for alcoholic and non-alcoholic liver fibrosis and cirrhosis. Cochrane Database Syst. Rev. 3, CD002148 (2005).
Nelson, D. R. et al. Long-term interleukin 10 therapy in chronic hepatitis C patients has a proviral and anti-inflammatory effect. Hepatology 38, 859–868 (2003).
Pockros, P. J. et al. Final results of a double-blind, placebo-controlled trial of the antifibrotic efficacy of interferon-γ1b in chronic hepatitis C patients with advanced fibrosis or cirrhosis. Hepatology 45, 569–578 (2007).
Colmenero, J. et al. Effects of losartan on hepatic expression of nonphagocytic NADPH oxidase and fibrogenic genes in patients with chronic hepatitis C. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G726–G734 (2009).
Thomas, J. A. et al. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration and function. Hepatology 53, 2003–2015 (2011).
Sakaida, I. et al. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology 40, 1304–1311 (2004).
Meier, R. P. et al. Microencapsulated human mesenchymal stem cells decrease liver fibrosis in mice. J. Hepatol. 62, 634–641 (2015).
Houlihan, D. D. & Newsome, P. N. Critical review of clinical trials of bone marrow stem cells in liver disease. Gastroenterology 135, 438–450 (2008).
Salama, H. et al. Autologous hematopoietic stem cell transplantation in 48 patients with end-stage chronic liver diseases. Cell Transplant. 19, 1475–1486 (2010).
Aldridge, V. et al. Human mesenchymal stem cells are recruited to injured liver in a β1-integrin and CD44 dependent manner. Hepatology 56, 1063–1073 (2012).
Moore, J. K., Stutchfield, B. M. & Forbes, S. J. Systematic review: the effects of autologous stem cell therapy for patients with liver disease. Aliment. Pharmacol. Ther. 39, 673–685 (2014).
Acknowledgements
This paper presents independent research supported by the Birmingham National Institute for Health Research (NIHR) Liver Biomedical Research Unit based at the University Hospital Birmingham NHS Foundation Trust and the University of Birmingham. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. P.N.N. is supported by the NIHR Biomedical Research Unit.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Forbes, S., Newsome, P. Liver regeneration — mechanisms and models to clinical application. Nat Rev Gastroenterol Hepatol 13, 473–485 (2016). https://doi.org/10.1038/nrgastro.2016.97
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2016.97
This article is cited by
-
Aging aggravates acetaminophen-induced acute liver injury and inflammation through inordinate C/EBPα-BMP9 crosstalk
Cell & Bioscience (2023)
-
Mangiferin relieves CCl4-induced liver fibrosis in mice
Scientific Reports (2023)
-
Mitochondria-derived H2O2 triggers liver regeneration via FoxO3a signaling pathway after partial hepatectomy in mice
Cell Death & Disease (2023)
-
DNA damage repair-related gene signature for identifying the immune status and predicting the prognosis of hepatocellular carcinoma
Scientific Reports (2023)
-
Influence of cholestasis on portal vein embolization-induced hypertrophy of the future liver remnant
Langenbeck's Archives of Surgery (2023)