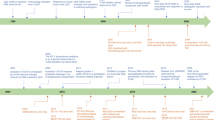
Key Points
-
Single-stranded DNA containing CpG motifs triggers an innate immune response that is characterized by the production of polyreactive immunoglobulin and the production of T helper 1 (TH1)-type and pro-inflammatory cytokines, and chemokines.
-
CpG DNA interacts with Toll-like receptor 9 (TLR9) in the endosomal vesicles of human B cells and plasmacytoid dendritic cells, initiating a signalling cascade that proceeds through myeloid differentiation primary response gene 88 (MYD88) and culminates in the translocation of nuclear factor-κB (NF-κB) from the cytoplasm to the nucleus. The subsequent cytokine response indirectly activates various other cell types, including natural killer cells, T cells and macrophages.
-
Human immune cells are stimulated by three structurally distinct classes of CpG oligodeoxynucleotide (ODN). Different types of immune response are triggered by each class of ODN.
-
The innate immune response that is triggered by CpG ODNs improves host resistance to a wide range of pathogenic bacteria, viruses and parasites.
-
By facilitating the production of TH1-type and pro-inflammatory cytokines, and promoting the functional maturation of professional antigen-presenting cells, CpG ODNs accelerate and boost the generation of antigen-specific immunity when co-administered with vaccines.
-
By promoting TH1-biased immunity, CpG ODNs dampen the development of TH2-cell-mediated allergic responses. Reduced allergen-specific IgE production and improved lung function accompany the administration of CpG ODNs with allergen.
-
CpG DNA has the potential to worsen organ-specific autoimmune disease and cause other pathological changes when administered repeatedly at a high dose. However, the regimens that are required to achieve the therapeutic effects described above have not caused adverse events in normal animals or humans.
-
CpG ODNs have been administered safely to more than 500 individuals. Preclinical and clinical results indicate that these agents will be of therapeutic value in the treatment of allergic disorders and for improving host immunity against infectious pathogens.
Abstract
Synthetic oligodeoxynucleotides (ODNs) that contain immunostimulatory CpG motifs trigger an immunomodulatory cascade that involves B and T cells, natural killer cells and professional antigen-presenting cells. The response to CpG ODNs skews the host's immune milieu in favour of T helper 1 (TH1)-cell responses and pro-inflammatory cytokine production — an effect that underlies their use as immunoprotective agents, vaccine adjuvants and anti-allergens. Preclinical studies provide evidence that CpG ODNs are effective for each of these uses. Ongoing clinical studies indicate that CpG ODN use is safe in humans, and that they modulate the immune response to co-administered allergens and vaccines.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Marrack, P. & Kappler, J. Subversion of the immune system by pathogens. Cell 76, 323–332 (1994).
Medzhitov, R. & Janeway, C. A. Innate immunity, impact on the adaptive immune response. Curr. Opin. Immunol. 9, 4–9 (1997).
Medzhitov, R. & Janeway, C. A. Innate immunity, the virtues of a nonclonal system of recognition. Cell 91, 295–298 (1998).
Paul, W. E. (ed.) in Fundamental Immunology 1–20 (Raven Press, New York, 2003).
Underhill, D. M. & Ozinsky, A. Toll-like receptors, key mediators of microbe detection. Curr. Opin. Immunol. 14, 103–110 (2002).
Vasselon, T. & Detmers, P. A. Toll receptors, a central element in innate immune responses. Infect. Immun. 70, 1033–1041 (2002).
Razin, A. & Friedman, J. DNA methylation and its possible biological roles. Prog. Nucl. Acid Res. 25, 33–52 (1981).
Cardon, L. R., Burge, C., Clayton, D. A. & Karlin, S. Pervasive CpG suppression in animal mitochondrial genomes. Proc. Natl Acad. Sci. USA 91, 3799–3803 (1994).
Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000). This paper shows that Toll-like receptor 9 (TLR9) mediates CpG recognition in mice.
Takeshita, F. et al. Cutting Edge, role of toll-like receptor 9 in CpG DNA-induced activation of human cells. J. Immunol. 167, 3555–3558 (2001). The authors show that TLR9 mediates CpG recognition in humans, and that CpG DNA co-localizes with TLR9 in endosomal vesicles.
Bauer, S. et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl Acad. Sci. USA 98, 9237–9242 (2001).
Wagner, H. Bacterial CpG-DNA activates immune cells to signal 'infectious danger'. Adv. Immunol. 73, 329–368 (1999).
Yamamoto, S. et al. Antitumor effect of nucleic acid fraction from bacteria. Proc. Jpn. Soc. Immunol. 48, 272–281 (1989).
Yamamoto, S. et al. Unique palindromic sequences in synthetic oligonucleotides are required to induce IFN and augment IFN-mediated natural killer activity. J. Immunol. 148, 4072–4076 (1992).
Krieg, A. M. et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374, 546–548 (1995). This work was the first to identify unmethylated CpG motifs as crucial mediators of immune activation. Studies showed that B cells responded to CpG motifs by proliferating and secreting immunoglobulin.
Klinman, D. M., Yi, A., Beaucage, S. L., Conover, J. & Krieg, A. M. CpG motifs expressed by bacterial DNA rapidly induce lymphocytes to secrete IL-6, IL-12 and IFNγ. Proc. Natl Acad. Sci. USA 93, 2879–2883 (1996). The first work to establish that unmethylated CpG motifs elicited a complex immunomodulatory cascade that included the production of T helper 1 (T H 1)-type and pro-inflammatory cytokines.
Jahrsdorfer, B. & Weiner, G. J. CpG oligodeoxynucleotides for immune stimulation in cancer immunotherapy. Curr. Opin. Investig. Drugs 4, 686–690 (2003).
Carpentier, A. F., Auf, G. & Delattre, J. Y. CpG-oligonucleotides for cancer immunotherapy, review of the literature and potential applications in malignant glioma. Front Biosci. 8, 115–127 (2003).
Ishii, K. J. et al. Potential role of phosphatidylinositol 3 kinase, rather than DNA-dependent protein kinase, in CpG DNA-induced immune activation. J. Exp. Med. 196, 269–274 (2002).
Yi, A. K. et al. CpG motifs in bacterial DNA activate leukocytes through the pH-dependent generation of reactive oxygen species. J. Immunol. 160, 4755–4761 (1998).
Hacker, H. et al. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 17, 6230–6540 (1998).
Takeshita, F., Ishii, K. J., Ueda, A., Ishigatsubo, Y. & Klinman, D. M. Positive and negative regulatory elements contribute to CpG oligonucleotide-mediated regulation of human IL-6 gene expression. Eur. J. Immunol. 30, 108–116 (2000).
Yamamoto, M. et al. Cutting edge, a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-β promoter in the Toll-like receptor signaling. J. Immunol. 169, 6668–6672 (2002).
Takeshita, F. & Klinman, D. M. CpG ODN-mediated regulation of IL-12 p40 transcription. Eur. J. Immunol. 30, 1967–1976 (2000).
Hacker, H. et al. Immune cell activation by bacterial CpG-DNA through myeroid differentiation marker 88 and tumor necrosis factor receptor-associated factor (TRAF)6. J. Exp. Med. 192, 595–600 (2000). This paper describes much of the intracellular signalling cascade that is triggered by the interaction of CpG DNA with TLR9.
Aderem, A. & Ulevitch, R. J. Toll-like receptors in the induction of the innate immune response. Nature 406, 782–787 (2000).
Ishii, K. J. et al. CpG-activated plasmacytoid dendritic cells protect against lethal Listeria monocytogenes infection. J. Immunol. (in the press).
Gursel, M., Verthelyi, D., Gursel, I., Ishii, K. J. & Klinman, D. M. Differential and competitive activation of human immune cells by distinct classes of CpG oligodeoxynucleotides. J. Leuk. Biol. 71, 813–820 (2002).
Hornung, V. et al. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168, 4531–4537 (2002).
Sun, S., Zhang, X., Tough, D. F. & Sprent, J. Type I interferon-mediated stimulation of T cells by CpG DNA. J. Exp. Med. 188, 2335–2342 (1998).
Stacey, K. J., Sweet, M. J. & Hume, D. A. Macrophages ingest and are activated by bacterial DNA. J. Immunol. 157, 2116–2120 (1996).
Ballas, Z. D., Rasmussen, W. L. & Krieg, A. M. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J. Immunol. 157, 1840–1847 (1996).
Roman, M. et al. Immunostimulatory DNA sequences function as T helper-1 promoting adjuvants. Nature Med. 3, 849–854 (1997).
Halpern, M. D., Kurlander, R. J. & Pisetsky, D. S. Bacterial DNA induces murine interferon-γ production by stimulation of IL-12 and tumor necrosis factor-α. Cell Immunol. 167, 72–78 (1996).
Rankin, R. et al. CpG motif identification for veterinary and laboratory species demonstrates that sequence recognition is highly conserved. Antisense Nucl. Acid Drug Dev. 11, 333–340 (2001).
Verthelyi, D., Ishii, K. J., Gursel, M., Takeshita, F. & Klinman, D. M. Human peripheral blood cells differentially recognize and respond to two distinct CpG motifs. J. Immunol. 166, 2372–2377 (2001). This is the first work to demonstrate that distinct types of CpG oligodeoxynucleotide (ODN) exist, and that human immune cells are differentially responsive to these different ODNs.
Krug, A. et al. Identification of CpG oligonucleotide sequences with high induction of IFNα/β in plasmacytoid dendritic cells. Eur. J. Immunol. 31, 2154–2163 (2001).
Kadowaki, N. et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194, 863–869 (2001).
Krug, A. et al. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur. J. Immunol. 31, 3026–3037 (2001).
Bauer, M. et al. Bacterial CpG DNA triggers activation and maturation of human CD11c−, CD123+ dendritic cells. J Immunol. 166, 5000–5007 (2001).
Hartmann, G. et al. Rational design of new CpG oligonucleotides that combine B cell activation with high IFN-α induction in plasmacytoid dendritic cells. Eur. J. Immunol. 33, 1633–1641 (2003).
Marshall, J. D. et al. Identification of a novel CpG DNA class and motif that optimally stimulate B cell and plasmacytoid dendritic cell functions. J. Leukoc. Biol. 73, 781–792 (2003).
Hartmann, G. & Krieg, A. M. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J. Immunol. 164, 944–952 (2000).
Verthelyi, D. et al. CpG oligodeoxynucleotides as vaccine adjuvants in primates. J. Immunol. 168, 1659–1663 (2002).
Davis, H. L. et al. CpG DNA overcomes hyporesponsiveness to hepatitis B vaccine in orangutans. Vaccine 18, 1920–1924 (2000).
Verthelyi, D. & Klinman, D. M. Immunoregulatory activity of CpG oligonucleotides in humans and nonhuman primates. Clin. Immunol. 109, 64–71 (2003).
Broide, D. H. et al. Systemic administration of immunostimulatory DNA sequences mediates reversible inhibition of TH2 responses in a mouse model of asthma. J. Clin. Immunol. 21, 175–182 (2001).
Kanellos, T. S. et al. Mammalian granulocyte–macrophage colony stimulating factor and some CpG motifs have an effect on the immunogenicity of DNA and subunit vaccines in fish. Immunology 96, 507–510 (1999).
Gomis, S. et al. Protection of chickens against Escherichia coli infections by DNA containing CpG motifs. Infect. Immun. 71, 857–863 (2003).
Pyles, R. B. et al. Use of immunostimulatory sequence-containing oligonucleotides as topical therapy for genital herpes simplex virus type 2 infection. J. Virol. 76, 11387–11396 (2002).
Ashkar, A. A., Bauer, S., Mitchell, W. J., Vieira, J. & Rosenthal, K. L. Local delivery of CpG oligodeoxynucleotides induces rapid changes in the genital mucosa and inhibits replication, but not entry, of herpes simplex virus type 2. J. Virol. 77, 8948–8956 (2003).
Klinman, D. M., Conover, J. & Coban, C. Repeated administration of synthetic oligodeoxynucleotides expressing CpG motifs provides long-term protection against bacterial infection. Infect. Immun. 67, 5658–5663 (1999).
Krieg, A. M., Homan, L. L., Yi, A. K. & Harty, J. T. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J. Immunol. 161, 2428–2434 (1998).
Walker, P. S. et al. Immunostimulatory oligodeoxynucleotides promote protective immunity and provide systemic therapy for leishmaniasis via IL-12 and IFN-γ dependent mechanisms. Proc. Natl Acad. Sci. USA 96, 6970–6975 (1999).
Elkins, K. L., Rhinehart-Jones, T. R., Stibitz, S., Conover, J. S. & Klinman, D. M. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J. Immunol. 162, 2291–2298 (1999).
Zimmermann, S. et al. CpG oligodeoxynucleotides trigger protective and curative TH1 responses in lethal murine Leishmaniasis. J. Immunol. 160, 3627–3630 (1998). This work was the first to establish that CpG ODNs could be used to increase host resistance to infectious pathogens.
Klinman, D. M. Therapeutic applications of CpG-containing oligodeoxynucleotides. Antisense Nucl. Acid Drug Dev. 8, 181–184 (1998).
Chiaramonte, M. G., Hesse, M., Cheever, A. W. & Wynn, T. A. CpG Oligonucleotides can prophylactically immunize against TH2-mediated schistosome egg induced pathology by an IL-12 independent mechanism. J. Immunol. 164, 973–985 (2000).
Yamamoto, T., Yamamoto, S., Katoaka, T. & Tokunaga, T. Lipofection of synthetic oligodeoxyribonucleotide having a palindromic sequence of AACGTT to murine splenocytes enhances IFN production and natural killer activity. Microbiol. Immunol. 38, 831–836 (1994).
Klinman, D. M., Barnhart, K. M. & Conover, J. CpG motifs as immune adjuvants. Vaccine 17, 19–25 (1999).
Pacanowski, J. et al. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood 98, 3016–3021 (2001).
Azzoni, L. et al. Sustained impairment of IFNγ secretion in suppressed HIV-infected patients despite mature NK cell recovery, evidence for a defective reconstitution of innate immunity. J. Immunol. 168, 5764–5770 (2002).
Chehimi, J. et al. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J. Immunol. 168, 4796–4801 (2002).
Corbett, E. L. et al. HIV-1/AIDS and the control of other infectious diseases in Africa. Lancet 359, 2177–2187 (2002).
Verthelyi, D. et al. CpG oligodeoxynucleotides protect normal and SIV-infected macaques from Leishmania infection. J. Immunol. 170, 4717–4723 (2003).
Weinberg, E. D. Pregnancy-associated depression of cell-mediated immunity. Rev. Infect. Dis. 6, 814–831 (1984).
Priddy, K. D. Immunologic adaptations during pregnancy. J. Obstet. Gynecol. Neonatal Nurs. 26, 388–394 (1997).
Wegmann, T. G., Lin, H., Guilbert, L. & Mosmann, T. R. Bidirectional cytokine interactions in the maternal–fetal relationship, is successful pregnancy a TH2 phenomenon? Immunol. Today 14, 353–356 (1993).
Lin, H., Mosmann, T. R., Guilbert, L., Tuntipopipat, S. & Wegmann, T. G. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J. Immunol. 151, 4562–4573 (1993).
Raghupathy, R. TH1-type immunity is incompatible with successful pregnancy. Immunol. Today 18, 478–482 (1997).
Sano, M., Mitsuyama, M., Watanabe, Y. & Nomoto, K. Impairment of T cell-mediated immunity to Listeria monocytogenes in pregnant mice. Microbiol. Immunol. 30, 165–176 (1986).
Krishnan, L. et al. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigen-specific IFN-γ response and increased production of T helper 2 cytokines. J. Immunol. 156, 644–652 (1996).
Ito, S., Ishii, K., Shirot, H. & Klinman, D. M. CpG oligodeoxynucleotides improve the survival of pregnant and fetal mice following Listeria monocytogenes infection. Infect. Immunol. (in the press).
Amaral, V. F. et al. Leishmania amazonensis: the asian rhesus macaques (Macaca mulata) as an experimental model for the study of cutaneous leishmaniasis. Exp. Parasitol. 82, 34–44 (1996).
Equils, O. et al. Toll-like receptor 2 (TLR2) and TLR9 signaling results in HIV-long terminal repeat trans-activation and HIV replication in HIV-1 transgenic mouse spleen cells, implications of simultaneous activation of TLRs on HIV replication. J. Immunol. 170, 5159–5164 (2003).
Olbrich, A. R., Schimmer, S. & Dittmer, U. Preinfection treatment of resistant mice with CpG oligodeoxynucleotides renders them susceptible to friend retrovirus-induced leukemia. J. Virol. 77, 10658–10662 (2003).
Gursel, I., Gursel, M., Ishii, K. J. & Klinman, D. M. Sterically stabilized cationic liposomes improve the uptake and immunostimulatory activity of CpG oligonucleotides. J. Immunol. 167, 3324–3328 (2001).
Davis, H. L. et al. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J. Immunol. 160, 870–876 (1998).
Shirota, H. et al. Novel roles of CpG oligodeoxynucleotides as a leader for the sampling and presentation of CpG-tagged antigen by dendritic cells. J. Immunol. 167, 66–74 (2001).
Moldoveanu, Z., Love-Homan, L., Huang, W. Q. & Krieg, A. M. CpG DNA, a novel immune enhancer for systemic and mucosal immunization with influenza virus. Vaccine 1216–1224 (1998).
Kovarik, J. et al. CpG oligonucleotides can cirmcuvent the TH2 polorization of neonatal responses to vaccines but fail to fully redirect TH2 responses established by neonatal priming. J. Immunol. 162, 1611–1617 (1999).
McCluskie, M. J. & Davis, H. L. CpG DNA is a potent enhancer of systemic and mucosal immune responses against hepatitis B surface antigen with intranasal administration to mice. J. Immunol. 161, 4463–4466 (1998).
Brazolot Millan, C. L., Weeratna, R., Krieg, A. M., Siegrist, C. A. & Davis, H. L. CpG DNA can induce strong TH1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. Proc. Natl Acad. Sci. USA 95, 15553–15558 (1998).
Eastcott, J. W. et al. Oligonucleotide containing CpG motifs enhances immune response to mucosally or systemically administered tetanus toxoid. Vaccine 19, 1636–1642 (2001).
Branda, R. F. et al. Amplification of antibody production by phosphorothioate oligodeoxynucleotides. J. Lab. Clin. Med. 128, 329–338 (1996).
Horner, A. A. et al. Immunostimulatory DNA is a potent mucosal adjuvant. Cell. Immunol. 190, 77–82 (1998).
McCluskie, M. J. & Davis, H. L. Oral, intrarectal and intranasal immunizations using CpG and non-CpG oligodeoxynucleotides as adjuvants. Vaccine 19, 413–422 (2000).
Jones, T. R. et al. Synthetic oligodeoxynucleotides containing CpG motifs enhance immunogenic vaccine in Aotus monkeys. Vaccine 17, 3065–3071 (1999). This report demonstrates that CpG ODNs designed for human use could act as immune adjuvants in non-human primates.
Klinman, D. M., Xie, H., Little, S. F., Currie, D. & Ivins, B. CpG Oligonucleotides improve the protective immune response induced by the anthrax vaccination of rhesus macaques. Vaccine (in the press).
von Stebut, E. et al. Leishmania major infected murine langerhans cell-like dendritic cells from susceptible mice release IL-12 after infection and vaccinate against experimental cutaneous leishmaniasis. Eur. J. Immunol. 30, 3498–3506 (2000).
Prince, G. A. et al. Immunoprotective activity and safety of a respiratory syncytial virus vaccine, mucosal delivery of fusion glycoprotein with a CpG oligodeoxynucleotide adjuvant. J. Virol. 77, 13156–13160 (2003).
Davis, H. L. Use of CpG DNA for enhancing specific immune responses. Curr. Top. Microbiol. Immunol. 247, 171–184 (2000).
Sears, M. R. Worldwide trends in asthma mortality. Bull. Int. Union Tuberc. Lung Dis. 66, 79–83 (1991).
Nakajima, H. et al. CD4+ T-lymphocytes and interleukin-5 mediate antigen-induced eosinophil infiltration into the mouse trachea. Am. Rev. Respir. Dis. 146, 374–377 (1992).
Robinson, D. S. et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 326, 298–304 (1992).
Nakajima, H., Iwamoto, I. & Yoshida, S. Aerosolized recombinant interferon-γ prevents antigen-induced eosinophil recruitment in mouse trachea. Am. Rev. Respir. Dis. 148, 1102–1104 (1993).
Sur, S. et al. Immunomodulatory effects of IL-12 on allergic lung inflammation depend on timing of doses. J. Immunol. 157, 4173–4180 (1996).
Shirakawa, T., Enomoto, T., Shimazu, S. & Hopkin, J. M. The inverse association between tuberculin responses and atopic disorder. Science 275, 77–79 (1997).
Erb, K. J., Holloway, J. W., Sobeck, A., Mol, l H. & Le Gros, G. Infection of mice with Mycobacterium bovis-Bacillus Calmette-Guerin (BCG) suppresses allergen-induced airway eosinophilia. J. Exp. Med. 187, 561–569 (1998).
Sur, S. et al. Long-term prevention of allergic lung inflammation in a mouse model of asthma by CpG oligodeoxynucleotides. J. Immunol. 162, 6284–6291 (1999).
Kline, J. N. et al. Modulation of airway inflammation by CpG oligodeoxynucleotides in a murine model of asthma. J. Immunol. 160, 2555–2559 (1998). This work was the first to establish that the combination of CpG ODNs plus allergen had a beneficial effect on allergic asthma.
Santeliz, J. V., Van Nest, G., Traquina, P., Larsen, E. & Wills-Karp, M. Amb a 1-linked CpG oligodeoxynucleotides reverse established airway hyperresponsiveness in a murine model of asthma. J. Allergy Clin. Immunol. 109, 455–462 (2002).
Horner, A. A. et al. Immunostimulatory DNA inhibits IL-4-dependent IgE synthesis by human B cells. J. Allergy Clin. Immunol. 108, 417–423 (2001).
Coyle, A. J. et al. Central role of immunoglobulin (Ig) E in the induction of lung eosinophil infiltration and T helper 2 cell cytokine production, inhibition by a non-anaphylactogenic anti-IgE antibody. J. Exp. Med. 183, 1303–1310 (1996).
Snapper, C. M., Peschel, C. & Paul, W. E. Interferon-γ stimulates IgG2a secretion by murine B cells stimulated with bacterial lipopolysaccharide. J. Immunol. 140, 2121–2130 (1988).
Tighe, H. et al. Conjugation of protein to immunostimulatory DNA results in a rapid, long-lasting and potent induction of cell-mediated and humoral immunity. Eur. J. Immunol. 30, 1939–1947 (2000).
Tighe, H. et al. Conjugation of immunostimulatory DNA to the short ragweed allergen amb a1 enhances its immunogenicity and reduces its allergenicity. J. Allergy Clin. Immunol. 106, 124–134 (2000).
Horner, A. A. et al. DNA-based vaccination reduces the risk of lethal anaphylactic hypersensitivity in mice. J. Allergy Clin. Immunol. 106, 349–356 (2000).
Horner, A. A., Takabaysahi, K., Zubeldia, J. M. & Raz, E. Immunostimulatory DNA-based therapeutics for experimental and clinical allergy. Allergy 57 (Suppl. 72), 24–29 (2002).
Horner, A. A. et al. Optimized conjugation ratios lead to allergen immunostimulatory oligodeoxynucleotide conjugates with retained immunogenicity and minimal anaphylactogenicity. J. Allergy Clin. Immunol. 110, 413–420 (2002).
Horner, A. A. & Raz, E. Immunostimulatory sequence oligodeoxynucleotide-based vaccination and immunomodulation, two unique but complementary strategies for the treatment of allergic diseases. J. Allergy Clin. Immunol. 110, 706–712 (2002).
Gilkeson, G. S., Riuz, P., Howell, D., Lefkowith, J. B. & Pisetsky, D. S. Induction of immune-mediated glomerulonephritis in normal mice immunized with bacterial DNA. Clin. Immunol. Immunopathol. 68, 283–292 (1993).
Gilkeson, G. S., Pippen, A. M. & Pisetsky, D. S. Induction of cross-reactive anti-dsDNA antibodies in preautoimmune NZB/NZW mice by immunization with bacterial DNA. J. Clin. Invest. 95, 1398–1402 (1995).
Steinberg, A. D., Krieg, A. M., Gourley, M. F. & Klinman, D. M. Theoretical and experimental approaches to generalized autoimmunity. Immunol. Rev. 118, 129–163 (1990).
Klinman, D. M. Polyclonal B cell activation in lupus-prone mice precedes and predicts the development of autoimmune disease. J. Clin. Invest. 86, 1249–1254 (1990).
Linker-Israeli, M. et al. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. J. Immunol. 147, 117–123 (1991).
Krieg, A. M. CpG DNA, a pathogenic factor in systemic lupus erythematosus? J. Clin. Immunol. 15, 284–292 (1995).
Yi, A. -K., Hornbeck, P., Lafrenz, D. E. & Krieg, A. M. CpG DNA rescue of murine B lymphoma cells from anti-IgM induced growth arrest and programmed cell death is associated with increased expression of c-Myc and Bcl-xl. J. Immunol. 157, 4918–4925 (1996).
Mor, G. et al. Do DNA vaccines induce autoimmune disease? Hum. Gene Ther. 8, 293–300 (1997).
Katsumi, A. et al. Humoral and cellural immunity to an encoded protein induced by direct DNA injection. Hum. Gene Ther. 5, 1335–1339 (1994).
Gilkeson, G. S. et al. Effects of bacterial DNA on cytokine production by (NZB/NZW)F1 mice. J. Immunol. 161, 3890–3895 (1998).
Segal, B. M., Klinman, D. M. & Shevach, E. M. Microbial products induce autoimmune disease by an IL-12 dependent process. J. Immunol. 158, 5087–5091 (1997).
Segal, B. M., Chang, J. T. & Shevach, E. M. CpG oligonucleotides are potent adjuvants for the activation of autoreactive encephalotogenic T cells in vivo. J. Immunol. 164, 5683–5688 (2000).
Bachmaier, K. et al. Chlamydia infections and heart disease linked through antigenic mimicry. Science 283, 1335–1339 (1999).
Zeuner, R. A., Verthelyi, D., Gursel, M., Ishii, K. J. & Klinman, D. M. Influence of stimulatory and suppressive DNA motifs on host susceptibility to inflammatory arthritis. Arthritis Rheum. 48, 1701–1707 (2003).
Sparwasser, T. et al. Bacterial DNA causes septic shock. Nature 386, 336–338 (1997).
Cowdery, J. S., Chace, J. H., Yi, A. -K. & Krieg, A. M. Bacterial DNA induces NK cells to produce IFNγ in vivo and increases the toxicity of lipopolysaccharides. J. Immunol. 156, 4570–4575 (1996).
Hartmann, G., Krug, A., Waller, K. & Endres, S. Oligodeoxynucleotides enhance LPS-stimulated synthesis of TNF, dependence on phosphorothioate modification and reversal by heparin. Mol. Med. 2, 429–438 (1996).
Heikenwalder, M. et al. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nature Med. 10, 187–192 (2004).
Klinman, D. M. et al. DNA vaccines, safety and efficacy issues. Springer Semin. Immunopathol. 19, 245–256 (1997).
Wang, R. et al. Induction of antigen specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 282, 476–480 (1998).
Calarota, S. et al. Cellular cytotoxic response induced by DNA vaccination in HIV-1 infected patients. Lancet 351, 1320–1325 (1998).
Yacyshyn, B. R. et al. Dose ranging pharmacokinetic trial of high-dose alicaforsen (intercellular adhesion molecule-1 antisense oligodeoxynucleotide) (ISIS 2302) in active Crohn's disease. Aliment. Pharmacol. Ther. 16, 1761–1770 (2002).
Liu, M. A. DNA vaccines, a review. J. Intern. Med. 253, 402–410 (2003).
Halperin, S. A. et al. A phase I study of the safety and immunogenicity of recombinant hepatitis B surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide adjuvant. Vaccine 21, 2461–2467 (2003).
Klinman, D. M. et al. Immunotherapeutic applications of CpG-containing oligodeoxynucleotides. Drug News Perspect. 13, 289–296 (2000).
Klinman, D. M. CpG DNA as a vaccine adjuvant. Expert Rev. Vacc. 2, 305–315 (2003).
Magone, M. T., Chan, C. C., Beck, L., Whitcup, S. M. & Raz, E. Systemic or mucosal administration of immunostimulatory DNA inhibits early and late phases of murine allergic conjunctivitis. Eur. J. Immunol. 30, 1841–1850 (2000).
von Hunolstein, C. et al. The adjuvant effect of synthetic oligodeoxynucleotide containing CpG motif converts the anti-Haemophilus influenzae type b glycoconjugates into efficient anti-polysaccharide and anti-carrier polyvalent vaccines. Vaccine 19, 3058–3066 (2001).
Temperton, N. J. et al. Enhancement of humoral immune responses to a human cytomegalovirus DNA vaccine, adjuvant effects of aluminum phosphate and CpG oligodeoxynucleotides. J. Med. Virol. 70, 86–90 (2003).
Al Mariri, A. et al. Protection of BALB/c mice against Brucella abortus 544 challenge by vaccination with bacterioferritin or P39 recombinant proteins with CpG oligodeoxynucleotides as adjuvant. Infect. Immun. 69, 4816–4822 (2001).
Hogarth, P. J., Jahans, K. J., Hecker, R., Hewinson, R. G. & Chambers, M. A. Evaluation of adjuvants for protein vaccines against tuberculosis in guinea pigs. Vaccine 21, 977–982 (2003).
Su, Z., Tam, M. F., Jankovic, D. & Stevenson, M. M. Vaccination with novel immunostimulatory adjuvants against blood-stage malaria in mice. Infect. Immun. 71, 5178–5187 (2003).
Mendez, S. et al. Coinjection with CpG-containing immunostimulatory oligodeoxynucleotides reduces the pathogenicity of a live vaccine against cutaneous Leishmaniasis but maintains its potency and durability. Infect. Immun. 71, 5121–5129 (2003).
Frank, F. M. et al. Use of a purified Trypanosoma cruzi antigen and CpG oligodeoxynucleotides for immunoprotection against a lethal challenge with trypomastigotes. Vaccine 22, 77–86 (2003).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
I hold a patent position on the discovery of CpG oligodeoxynucleotides.
Glossary
- PATHOGEN-ASSOCIATED MOLECULAR PATTERNS
-
(PAMPs). The innate immune system is stimulated by interaction with a highly conserved, but limited, set of molecular structures that are expressed by infectious microorganisms but absent (or rarely expressed) by the host. These PAMPs are recognized by a set of germline-encoded receptors, of which the family of Toll-like receptors is the best described.
- TH1/TH2 CELLS
-
Two functionally distinct subsets of CD4+ T cells. T helper 1 (TH1) cells produce type 1 cytokines (including interleukin-2, IL-2, and interferon-γ) that support macrophage activation, the generation of cytotoxic T cells and the production of opsonizing antibodies. TH2 cells produce type 2 cytokines (including IL-4, IL-5 and/or IL-13) that support B-cell activation, the production of non-opsonizing antibodies, allergic reactions and the expulsion of extracellular parasites.
- PLASMACYTOID DENDRITIC CELLS
-
(pDCs). A subset of DCs that were first described in humans and termed 'plasmacytoid' because of their microscopic appearance, which is similar to plasmablasts. In humans, these DCs can be derived from lineage-negative stem cells in peripheral blood and are the main producers of type I interferons (IFNs) in response to viral infections. Recent studies have identified a subset of type I IFN-producing DCs in mice, which are characterized by expression of B220 and Ly6C/G.
- TOXIC SHOCK
-
A state of circulatory collapse and end-organ failure that is associated with the release of tumour-necrosis factor by host mononuclear cells in response to an infectious challenge.
- LUPUS
-
(Also known as systemic lupus erythematosus, SLE). Lupus is an autoimmune disease that affects many tissues and cell types. Disease is characterized by the production of pathogenic autoantibodies that form immune complexes that can damage the kidneys and lungs. Multiple immune abnormalities, particularly associated with the increased activation of B and T cells, typify active disease.
- EXPERIMENTAL ALLERGIC ENCEPHALOMYELITIS
-
(EAE). An animal model of multiple sclerosis — a chronic demyelinating disease in humans. In animals, EAE is induced by injecting antigens derived from the myelin sheath of nerve cells, including myelin basic protein, proteolipid protein and myelin oligodendrocyte glycoprotein, together with a potent adjuvant.
- MOLECULAR MIMICRY
-
A mechanism for the induction of autoimmunity in which the host mounts an immune response against a protein or peptide expressed by a pathogen that resembles a determinant also present on self-tissue. The induction of a pathogen-specific immune response therefore results in a crossreactive response to the self-tissue that causes pathology.
Rights and permissions
About this article
Cite this article
Klinman, D. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol 4, 249–259 (2004). https://doi.org/10.1038/nri1329
Issue Date:
DOI: https://doi.org/10.1038/nri1329
This article is cited by
-
Vaccination with a combination of STING agonist-loaded lipid nanoparticles and CpG-ODNs protects against lung metastasis via the induction of CD11bhighCD27low memory-like NK cells
Experimental Hematology & Oncology (2024)
-
Gut health benefit and application of postbiotics in animal production
Journal of Animal Science and Biotechnology (2022)
-
Fabrication of manganese-coordinated polyphenol carbon dots for photothermal therapy and immune activation
Cancer Nanotechnology (2022)
-
Designer DNA nanostructures for viral inhibition
Nature Protocols (2022)
-
In situ vaccination using unique TLR9 ligand K3-SPG induces long-lasting systemic immune response and synergizes with systemic and local immunotherapy
Scientific Reports (2022)