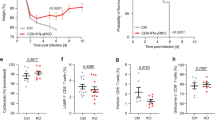
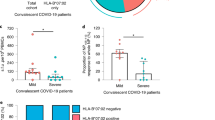
Abstract
At present, we only have indirect knowledge of the protective role of antigen-specific T cells in human viral infections, and it has been difficult to show a direct correlation between quantitative and qualitative measures of T-cell immunity and virus-associated diseases. However, as described in this Opinion article, recent advances in the characterization of T-cell functions and in the development of standardized T-cell assays have led to the identification of distinct functional signatures of T-cell responses that correlate with levels of viral replication and disease activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Klenerman, P. & Hill, A. T cells and viral persistence: lessons from diverse infections. Nature Immunol. 6, 873–879 (2005).
Ahmed, R. & Gray, D. Immunological memory and protective immunity: understanding their relation. Science 272, 54–60 (1996).
Cox, J. H. et al. Results of an ELISPOT proficiency panel conducted in 11 laboratories participating in international human immunodeficiency virus type 1 vaccine trials. AIDS Res. Hum. Retroviruses 21, 68–81 (2005).
Maecker, H. T. et al. Standardization of cytokine flow cytometry assays. BMC Immunol. 6, 13 (2005).
Lau, L. L., Jamieson, B. D., Somasundaram, T. & Ahmed, R. Cytotoxic T-cell memory without antigen. Nature 369, 648–652 (1994).
Murali-Krishna, K. et al. Counting antigen-specific CD8 T cells: a re-evaluation of bystander activation during viral infection. Immunity 8, 177–187 (1998).
Kaech, S. M. & Ahmed, R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nature Immunol. 2, 415–422 (2001).
Wherry, E. J. et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nature Immunol. 4, 225–234 (2003).
Wherry, E. J., Barber, D. L., Kaech, S. M., Blattman, J. N. & Ahmed, R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl Acad. Sci. USA 101, 16004–16009 (2004).
Sallusto, F. et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Champagne, P. et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410, 106–111 (2001).
Lyons, A. B. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J. Immunol. Methods 243, 147–154 (2000).
Hill, P. C. et al. ESAT-6/CFP-10 fusion protein and peptides for optimal diagnosis of Mycobacterium tuberculosis infection by ex vivo enzyme-linked immunospot assay in the Gambia. J. Clin. Microbiol. 43, 2070–2074 (2005).
Liebeschuetz, S. et al. Diagnosis of tuberculosis in South African children with a T-cell-based assay: a prospective cohort study. Lancet 364, 2196–2203 (2004).
Lalvani, A. et al. Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet 357, 2017–2021 (2001).
Chapman, A. L. et al. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis-specific T cells. AIDS 16, 2285–2293 (2002).
Lazarevic, V., Nolt, D. & Flynn, J. L. Long-term control of Mycobacterium tuberculosis infection is mediated by dynamic immune responses. J. Immunol. 175, 1107–1117 (2005).
Bunde, T. et al. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J. Exp. Med. 201, 1031–1036 (2005).
Sester, M. et al. Levels of virus-specific CD4 T cells correlate with cytomegalovirus control and predict virus-induced disease after renal transplantation. Transplantation 71, 1287–1294 (2001).
Fishman, J. A. & Rubin, R. H. Infection in organ-transplant recipients. N. Engl. J. Med. 338, 1741–1751 (1998).
Harari, A. et al. Analysis of HIV-1- and CMV-specific memory CD4 T-cell responses during primary and chronic infection. Blood 100, 1381–1387 (2002).
Ellefsen, K. et al. Distribution and functional analysis of memory antiviral CD8 T cell responses in HIV-1 and cytomegalovirus infections. Eur. J. Immunol. 32, 3756–3764 (2002).
Harari, A., Petitpierre, S., Vallelian, F. & Pantaleo, G. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood 103, 966–972 (2004).
Harari, A., Vallelian, F. & Pantaleo, G. Phenotypic heterogeneity of antigen-specific CD4 T cells under different conditions of antigen persistence and antigen load. Eur. J. Immunol. 34, 3525–3533 (2004).
Zimmerli, S. C. et al. HIV-1-specific IFN-γ/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc. Natl Acad. Sci. USA 102, 7239–7244 (2005).
Harari, A., Vallelian, F., Meylan, P. R. & Pantaleo, G. Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. J. Immunol. 174, 1037–1045 (2005).
Hamann, D. et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 186, 1407–1418 (1997).
Callan, M. F. et al. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein–Barr virus in vivo. J. Exp. Med. 187, 1395–1402 (1998).
Appay, V. et al. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192, 63–75 (2000).
Betts, M. R. et al. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75, 11983–11991 (2001).
Komanduri, K. V. et al. Direct measurement of CD4+ and CD8+ T-cell responses to CMV in HIV-1-infected subjects. Virology 279, 459–470 (2001).
Appay, V. et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nature Med. 8, 379–385 (2002).
Migueles, S. A. et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nature Immunol. 3, 1061–1068 (2002).
Addo, M. M. et al. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 77, 2081–2092 (2003).
Iyasere, C. et al. Diminished proliferation of human immunodeficiency virus-specific CD4+ T cells is associated with diminished interleukin-2 (IL-2) production and is recovered by exogenous IL-2. J. Virol. 77, 10900–10909 (2003).
Younes, S. A. et al. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J. Exp. Med. 198, 1909–1922 (2003).
Amyes, E. et al. Characterization of the CD4+ T cell response to Epstein–Barr virus during primary and persistent infection. J. Exp. Med. 198, 903–911 (2003).
Palmer, B. E., Boritz, E. & Wilson, C. C. Effects of sustained HIV-1 plasma viremia on HIV-1 Gag-specific CD4+ T cell maturation and function. J. Immunol. 172, 3337–3347 (2004).
Lichterfeld, M. et al. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J. Exp. Med. 200, 701–712 (2004).
Dunne, P. J. et al. Quiescence and functional reprogramming of Epstein–Barr virus (EBV)-specific CD8+ T cells during persistent infection. Blood 106, 558–565 (2005).
Betts, M. R. et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T-cells. Blood 7 Feb 2006 (doi:10.1182/blood-2005-12-4818).
Betts, M. R. et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 281, 65–78 (2003).
Betts, M. R. & Koup, R. A. Detection of T-cell degranulation: CD107a and b. Methods Cell. Biol. 75, 497–512 (2004).
Giudotti, L. G. & Chisari, F. V. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19, 65–91 (2001).
Komanduri, K. V. et al. Loss of cytomegalovirus-specific CD4+ T cell responses in human immunodeficiency virus type 1-infected patients with high CD4+ T cell counts and recurrent retinitis. J. Infect. Dis. 183, 1285–1289 (2001).
Kaech, S. M. et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nature Immunol. 4, 1191–1198 (2003).
Lang, K. S. et al. Inverse correlation between IL-7 receptor expression and CD8 T cell exhaustion during persistent antigen stimulation. Eur. J. Immunol. 35, 738–745 (2005).
Sze, D. M. et al. Clonal cytotoxic T cells are expanded in myeloma and reside in the CD8+CD57+CD28− compartment. Blood 98, 2817–2827 (2001).
van Baarle, D., Kostense, S., van Oers, M. H., Hamann, D. & Miedema, F. Failing immune control as a result of impaired CD8+ T-cell maturation: CD27 might provide a clue. Trends Immunol. 23, 586–591 (2002).
Weekes, M. P. et al. Large clonal expansions of human virus-specific memory cytotoxic T lymphocytes within the CD57+ CD28−CD8+ T-cell population. Immunology 98, 443–449 (1999).
Brenchley, J. M. et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101, 2711–2720 (2003).
Aandahl, E. M. et al. CD7 is a differentiation marker that identifies multiple CD8 T cell effector subsets. J. Immunol. 170, 2349–2355 (2003).
Liu, Z. et al. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the multicenter AIDS cohort study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 16, 83–92 (1997).
Roederer, M., Herzenberg, L. A. & Herzenberg, L. A. Changes in antigen densities on leukocyte subsets correlate with progression of HIV disease. Int. Immunol. 8, 1–11 (1996).
Van Baarle, D. et al. Lack of Epstein–Barr virus- and HIV-specific CD27− CD8+ T cells is associated with progression to viral disease in HIV-infection. AIDS 16, 2001–2011 (2002).
Hess, C. et al. HIV-1 specific CD8+ T cells with an effector phenotype and control of viral replication. Lancet 363, 863–866 (2004).
Paiardini, M. et al. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J. Immunol. 174, 2900–2909 (2005).
Doherty, P. C. & Christensen, J. P. Accessing complexity: the dynamics of virus-specific T cell responses. Annu. Rev. Immunol. 18, 561–592 (2000).
Sprent, J. & Surh, C. D. T cell memory. Annu. Rev. Immunol. 20, 551–579 (2002).
Semmo, N. et al. Preferential loss of IL-2-secreting CD4+ T helper cells in chronic HCV infection. Hepatology 41, 1019–1028 (2005).
Acknowledgements
This work was supported by research grants from the Swiss National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
CDC Infectious Disease Information
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Pantaleo, G., Harari, A. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nat Rev Immunol 6, 417–423 (2006). https://doi.org/10.1038/nri1840
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri1840
This article is cited by
-
Cumulative SARS-CoV-2 mutations and corresponding changes in immunity in an immunocompromised patient indicate viral evolution within the host
Nature Communications (2022)
-
Low-dose decitabine priming endows CAR T cells with enhanced and persistent antitumour potential via epigenetic reprogramming
Nature Communications (2021)
-
Dynamic metrics-based biomarkers to predict responders to anti-PD-1 immunotherapy
British Journal of Cancer (2019)
-
Pilot study on an innovative biosensor with a range of medical and surgical applications
BMC Research Notes (2018)
-
Evolution of T Cell Responses during Measles Virus Infection and RNA Clearance
Scientific Reports (2017)