Abstract
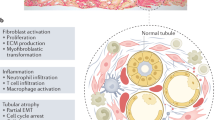
Obstructive nephropathy is a major cause of renal failure, particularly in newborn babies and children. After urinary tract obstruction, and under the influence of mechanical forces and cytokines produced by tubular cells and cells that have infiltrated the interstitium, resident fibroblasts undergo activation and myofibroblasts are generated from bone-marrow-derived cells, pericytes and endothelial cells. In addition, selected tubular epithelial cells can become fibroblast-like cells via epithelial–mesenchymal transition. This transition is characterized by downregulation of epithelial marker proteins such as E-cadherin, zonula occludens 1 and cytokeratin; loss of cell-to-cell adhesion; upregulation of mesenchymal markers including vimentin, α-smooth muscle actin and fibroblast-specific protein 1; basement membrane degradation; and migration to the interstitial compartment. All the events of epithelial–mesenchymal transition are strictly regulated by complex signaling pathways. Myofibroblasts and activated fibroblasts proliferate and produce large amounts of extracellular matrix, which accumulates in the tubular interstitium; together with tubular atrophy, this accumulation leads to interstitial fibrosis. This Review examines the molecular mechanisms of fibroblast activation and epithelial–mesenchymal transition, processes that seem to be promising targets for the prevention, or even reversal, of interstitial fibrosis and renal dysfunction associated with obstructive nephropathy.
Key Points
-
Obstructive nephropathy, a leading cause of chronic kidney disease in children, is characterized by inflammation, tubular atrophy and interstitial fibrosis
-
Activation of local fibroblasts and generation of myofibroblasts from epithelial cells (via epithelial–mesenchymal transition [EMT]), pericytes, endothelial cells and bone-marrow-derived cells are key processes in tubulointerstitial fibrosis
-
Data from rodent models of unilateral ureteral obstruction and in vitro studies have shed new light on the molecular mechanisms that underlie these processes
-
Fibroblast activation and EMT are induced by mechanical forces and cytokines such as transforming growth factor β, platelet-derived growth factor, fibroblast growth factor and activin A
-
EMT is strictly regulated by several signaling pathways that involve small GTPases, mitogen-activated protein kinases, phosphatidylinositol 3 kinase–Akt, glycogen synthase kinase 3β, integrin-linked kinase–PINCH and nuclear factor κB, among other molecules
-
Knowledge of the molecular mechanisms responsible for fibroblast activation and EMT is essential for the development of pharmacological strategies to prevent or treat interstitial fibrosis associated with obstructive nephropathy
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bohle, A., Strutz, F. & Müller, G. A. On the pathogenesis of chronic renal failure in primary glomerulopathies: a view from the interstitium. Exp. Nephrol. 2, 205–210 (1994).
Manucha, W. Biochemical-molecular markers in unilateral ureteral obstruction. Biocell 31, 1–12 (2007).
Lopez-Novoa, J. M. in The Aging Kidney in Health and Disease (eds Macias-Nuñez, J. F. et al.) 113–126 (Springer, New York, 2008).
Joosten, S. A., Sijpkens, Y. W., van Kooten, C. & Paul, L. C. Chronic renal allograft rejection: pathophysiologic considerations. Kidney Int. 68, 1–13 (2005).
Smith, J. M., Stablein, D. M., Munoz, R., Hebert, D. & McDonald, R. A. Contributions of the transplant registry: the 2006 annual report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS). Pediatr. Transplant. 11, 366–373 (2007).
Chevalier, R. L. Obstructive nephropathy: towards biomarker discovery and gene therapy. Nat. Clin. Pract. Nephrol. 2, 157–168 (2006).
Strutz, F. & Zeisberg, M. Renal fibroblasts and myofibroblasts in chronic kidney disease. J. Am. Soc. Nephrol. 17, 2992–2998 (2006).
Qi, W. et al. The renal cortical fibroblast in renal tubulointerstitial fibrosis. Int. J. Biochem. Cell Biol. 38, 1–5 (2006).
Strutz, F. How many different roads may a cell walk down in order to become a fibroblast? J. Am. Soc. Nephrol. 19, 2246–2248 (2008).
Roufosse, C. et al. Bone marrow-derived cells do not contribute significantly to collagen I synthesis in a murine model of renal fibrosis. J. Am. Soc. Nephrol. 17, 775–782 (2006).
Picard, N., Baum, O., Vogetseder, A., Kaissling, B. & Le Hir, M. Origin of renal myofibroblasts in the model of unilateral ureter obstruction in the rat. Histochem. Cell Biol. 130, 141–155 (2008).
Lin, S. L., Kisseleva, T., Brenner, D. A. & Duffield, J. S. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am. J. Pathol. 173, 1617–1627 (2008).
Zeisberg, E. M., Potenta, S. E., Sugimoto, H., Zeisberg, M. & Kalluri, R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J. Am. Soc. Nephrol. 19, 2282–2287 (2008).
Zeisberg, M. & Kalluri, R. Fibroblasts emerge via epithelial–mesenchymal transition in chronic kidney fibrosis. Front. Biosci. 13, 6991–6998 (2008).
Yamashita, S., Maeshima, A. & Nojima, Y. Involvement of renal progenitor tubular cells in epithelial-to-mesenchymal transition in fibrotic rat kidneys. J. Am. Soc. Nephrol. 16, 2044–2051 (2005).
Le Hir, M., Hegyi, I., Cueni-Loffing, D., Loffing, J. & Kaissling, B. Characterization of renal interstitial fibroblast-specific protein 1/S100A4-positive cells in healthy and inflamed rodent kidneys. Histochem. Cell Biol. 123, 335–346 (2005).
Kaissling, B. & Le Hir, M. The renal cortical interstitium: morphological and functional aspects. Histochem. Cell Biol. 130, 247–262 (2008).
Inoue, T., Plieth, D., Venkov, C. D., Xu, C. & Neilson, E. G. Antibodies against macrophages that overlap in specificity with fibroblasts. Kidney Int. 67, 2488–2493 (2005).
Desmouliere, A., Chaponnier, C. & Gabbiani, G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 13, 7–12 (2005).
El Chaar, M. et al. Cyclooxygenase-2 inhibitor decreases extracellular matrix synthesis in stretched renal fibroblasts. Nephron Exp. Nephrol. 100, e150–e155 (2005).
Wang, W., Koka, V. & Lan, H. Y. Transforming growth factor-β and Smad signalling in kidney diseases. Nephrology (Carlton) 10, 48–56 (2005).
Eitner, F. et al. PDGF-C is a proinflammatory cytokine that mediates renal interstitial fibrosis. J. Am. Soc. Nephrol. 19, 281–289 (2008).
Strutz, F. et al. Basic fibroblast growth factor expression is increased in human renal fibrogenesis and may mediate autocrine fibroblast proliferation. Kidney Int. 57, 1521–1538 (2000).
Hinz, B. Masters and servants of the force: the role of matrix adhesions in myofibroblast force perception and transmission. Eur. J. Cell Biol. 85, 175–181 (2006).
Lange-Sperandio, B. et al. Leukocytes induce epithelial to mesenchymal transition after unilateral ureteral obstruction in neonatal mice. Am. J. Pathol. 171, 861–871 (2007).
Huang, X. R., Chung, A. C., Wang, X. J., Lai, K. N. & Lan, H. Y. Mice overexpressing latent TGF-β1 are protected against renal fibrosis in obstructive kidney disease. Am. J. Physiol. Renal Physiol. 295, F118–F127 (2008).
Sato, M., Muragaki, Y., Saika, S., Roberts, A. B. & Ooshima, A. Targeted disruption of TGF-β1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J. Clin. Invest. 112, 1486–1494 (2003).
Tan, R. et al. Downregulation of SnoN expression in obstructive nephropathy is mediated by an enhanced ubiquitin-dependent degradation. J. Am. Soc. Nephrol. 17, 2781–2791 (2006).
Fukasawa, H. et al. Ubiquitin-dependent degradation of SnoN and Ski is increased in renal fibrosis induced by obstructive injury. Kidney Int. 69, 1733–1740 (2006).
Yamashita, S., Maeshima, A., Kojima, I. & Nojima, Y. Activin A is a potent activator of renal interstitial fibroblasts. J. Am. Soc. Nephrol. 15, 91–101 (2004).
Lee, D. B., Huang, E. & Ward, H. J. Tight junction biology and kidney dysfunction. Am. J. Physiol. Renal Physiol. 290, F20–F34 (2006).
Iwano, M. et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Invest. 110, 341–350 (2002).
Faulkner, J. L., Szcykalski, L. M., Springer, F. & Barnes, J. L. Origin of interstitial fibroblasts in an accelerated model of angiotensin II-induced renal fibrosis. Am. J. Pathol. 167, 1193–1205 (2005).
Rastaldi, M. P. et al. Epithelial–mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int. 62, 137–146 (2002).
Nishitani, Y. et al. Fibroblast-specific protein 1 is a specific prognostic marker for renal survival in patients with IgAN. Kidney Int. 68, 1078–1085 (2005).
Rossini, M. et al. Immunolocalization of fibroblast growth factor-1 (FGF-1), its receptor (FGFR-1), and fibroblast-specific protein-1 (FSP-1) in inflammatory renal disease. Kidney Int. 68, 2621–2628 (2005).
Hertig, A. et al. Risk factors for early epithelial to mesenchymal transition in renal grafts. Am. J. Transplant. 6, 2937–2946 (2006).
Vongwiwatana, A., Tasanarong, A., Rayner, D. C., Melk, A. & Halloran, P. F. Epithelial to mesenchymal transition during late deterioration of human kidney transplants: the role of tubular cells in fibrogenesis. Am. J. Transplant. 5, 1367–1374 (2005).
Boutet, A. et al. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J. 25, 5603–5613 (2006).
Wu, M. J. et al. Rapamycin attenuates unilateral ureteral obstruction-induced renal fibrosis. Kidney Int. 69, 2029–2036 (2006).
Li, Y., Dai, C., Wu, C. & Liu, Y. PINCH-1 promotes tubular epithelial-to-mesenchymal transition by interacting with integrin-linked kinase. J. Am. Soc. Nephrol. 18, 2534–2543 (2007).
Surendran, K., Schiavi, S. & Hruska, K. A. Wnt-dependent β-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J. Am. Soc. Nephrol. 16, 2373–2384 (2005).
Liu, Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J. Am. Soc. Nephrol. 15, 1–12 (2004).
Docherty, N. G. et al. Increased E-cadherin expression in the ligated kidney following unilateral ureteric obstruction. Kidney Int. 75, 205–213 (2009).
Butt, M. J., Tarantal, A. F., Jimenez, D. F. & Matsell, D. G. Collecting duct epithelial–mesenchymal transition in fetal urinary tract obstruction. Kidney Int. 72, 936–944 (2007).
Barrallo-Gimeno, A. & Nieto, M. A. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 132, 3151–3161 (2005).
Yoshino, J. et al. Snail1 is involved in the renal epithelial–mesenchymal transition. Biochem. Biophys. Res. Commun. 362, 63–68 (2007).
Yang, J. et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117, 927–939 (2004).
Kida, Y., Asahina, K., Teraoka, H., Gitelman, I. & Sato, T. Twist relates to tubular epithelial–mesenchymal transition and interstitial fibrogenesis in the obstructed kidney. J. Histochem. Cytochem. 55, 661–673 (2007).
Peinado, H., Olmeda, D. & Cano, A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat. Rev. Cancer 7, 415–428 (2007).
Park, S. M., Gaur, A. B., Lengyel, E. & Peter, M. E. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 22, 894–907 (2008).
Horiguchi, K. et al. Role of Ras signaling in the induction of Snail by transforming growth factor-β. J. Biol. Chem. 284, 245–253 (2009).
Choi, J., Park, S. Y. & Joo, C. K. Transforming growth factor-β1 represses E-cadherin production via Slug expression in lens epithelial cells. Invest. Ophthalmol. Vis. Sci. 48, 2708–2718 (2007).
Thiery, J. P. & Sleeman, J. P. Complex networks orchestrate epithelial–mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 7, 131–142 (2006).
Qiao, M., Sheng, S. & Pardee, A. B. Metastasis and Akt activation. Cell Cycle 7, 2991–2996 (2008).
Rodriguez-Pena, A. B. et al. Activation of Erk1/2 and Akt following unilateral ureteral obstruction. Kidney Int. 74, 196–209 (2008).
Jiang, Y. G. et al. Role of Wnt/β-catenin signaling pathway in epithelial–mesenchymal transition of human prostate cancer induced by hypoxia-inducible factor-1α. Int. J. Urol. 14, 1034–1039 (2007).
Huber, M. A. et al. NF-κB is essential for epithelial–mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Invest. 114, 569–581 (2004).
Pham, C. G. et al. Upregulation of Twist-1 by NF-κB blocks cytotoxicity induced by chemotherapeutic drugs. Mol. Cell Biol. 27, 3920–3935 (2007).
Peinado, H. & Cano, A. A hypoxic twist in metastasis. Nat. Cell Biol. 10, 253–254 (2008).
Barberà, M. J. et al. Regulation of Snail transcription during epithelial to mesenchymal transition of tumor cells. Oncogene 23, 7345–7354 (2004).
Chua, H. L. et al. NF-κB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene 26, 711–724 (2007).
McDonald, P. C., Fielding, A. B. & Dedhar, S. Integrin-linked kinase-—essential roles in physiology and cancer biology. J. Cell Sci. 121, 3121–3132 (2008).
Legate, K. R., Montañez, E., Kudlacek, O. & Fässler, R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat. Rev. Mol. Cell Biol. 7, 20–31 (2006).
Li, Y., Yang, J., Dai, C., Wu, C. & Liu, Y. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J. Clin. Invest. 112, 503–516 (2003).
Deckers, M. et al. The tumor suppressor Smad4 is required for transforming growth factor β-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. Cancer Res. 66, 2202–2209 (2006).
Phanish, M. K., Wahab, N. A., Colville-Nash, P., Hendry, B. M. & Dockrell, M. E. The differential role of Smad2 and Smad3 in the regulation of pro-fibrotic TGFβ1 responses in human proximal-tubule epithelial cells. Biochem. J. 393, 601–607 (2006).
Runyan, C. E., Schnaper, H. W. & Poncelet, A. C. The role of internalization in transforming growth factor β1-induced Smad2 association with Smad anchor for receptor activation (SARA) and Smad2-dependent signaling in human mesangial cells. J. Biol. Chem. 280, 8300–8308 (2005).
Inazaki, K. et al. Smad3 deficiency attenuates renal fibrosis, inflammation, and apoptosis after unilateral ureteral obstruction. Kidney Int. 66, 597–604 (2004).
Nawshad, A., Lagamba, D., Polad, A. & Hay, E. D. Transforming growth factor-β signaling during epithelial–mesenchymal transformation: implications for embryogenesis and tumor metastasis. Cells Tissues Organs 179, 11–23 (2005).
Zavadil, J. & Bottinger, E. P. TGF-β and epithelial-to-mesenchymal transitions. Oncogene 24, 5764–5774 (2005).
Postigo, A. A., Depp, J. L., Taylor, J. J. & Kroll, K. L. Regulation of Smad signaling through a differential recruitment of coactivators and corepressors by ZEB proteins. EMBO J. 22, 2453–2462 (2003).
Venkov, C. D. et al. A proximal activator of transcription in epithelial–mesenchymal transition. J. Clin. Invest. 117, 482–491 (2007).
Vidyasagar, A., Reese, S., Acun, Z., Hullett, D. & Djamali, A. HSP27 is involved in the pathogenesis of kidney tubulointerstitial fibrosis. Am. J. Physiol. Renal Physiol. 295, F707–F716 (2008).
Li, Y., Yang, J., Luo, J. H., Dedhar, S. & Liu, Y. Tubular epithelial cell dedifferentiation is driven by the helix-loop-helix transcriptional inhibitor Id1. J. Am. Soc. Nephrol. 18, 449–460 (2007).
Yang, J. et al. Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. J. Clin. Invest. 110, 1525–1538 (2002).
Cai, G. et al. Tissue inhibitor of metalloproteinase-1 exacerbated renal interstitial fibrosis through enhancing inflammation. Nephrol. Dial. Transplant. 23, 1861–1875 (2008).
Nishida, M. et al. MMP-2 inhibition reduces renal macrophage infiltration with increased fibrosis in UUO. Biochem. Biophys. Res. Commun. 354, 133–139 (2007).
Surendran, K., Simon, T. C., Liapis, H. & McGuire, J. K. Matrilysin (MMP-7) expression in renal tubular damage: association with Wnt4. Kidney Int. 65, 2212–2222 (2004).
Jorda, M. et al. Upregulation of MMP-9 in MDCK epithelial cell line in response to expression of the Snail transcription factor. J. Cell Sci. 118, 3371–3385 (2005).
Yanagisawa, M. et al. A p120 catenin isoform switch affects Rho activity, induces tumor cell invasion, and predicts metastatic disease. J. Biol. Chem. 283, 18344–18354 (2008).
Cavallaro, U. & Christofori, G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat. Rev. Cancer 4, 118–132 (2004).
Bellovin, D. I., Bates, R. C., Muzikansky, A., Rimm, D. L. & Mercurio, A. M. Altered localization of p120 catenin during epithelial to mesenchymal transition of colon carcinoma is prognostic for aggressive disease. Cancer Res. 65, 10938–10945 (2005).
Moriyama, T. & Nagatoya, K. The Rho-ROCK system as a new therapeutic target for preventing interstitial fibrosis. Drug News Perspect. 17, 29–34 (2004).
Fu, P. et al. Signaling mechanism of renal fibrosis in unilateral ureteral obstructive kidney disease in ROCK1 knockout mice. J. Am. Soc. Nephrol. 17, 3105–3114 (2006).
Patel, S. et al. RhoGTPase activation is a key step in renal epithelial mesenchymal transdifferentiation. J. Am. Soc. Nephrol. 16, 1977–1984 (2005).
Kaartinen, V., Haataja, L., Nagy, A., Heisterkamp, N. & Groffen, J. TGFβ3-induced activation of RhoA/Rho-kinase pathway is necessary but not sufficient for epithelio–mesenchymal transdifferentiation: implications for palatogenesis. Int. J. Mol. Med. 9, 563–570 (2002).
Guarino, M., Rubino, B. & Ballabio, G. The role of epithelial–mesenchymal transition in cancer pathology. Pathology 39, 305–318 (2007).
Grande, M. T. & López-Novoa, J. M. Therapeutical relevance of MAP-kinase inhibitors in renal diseases: current knowledge and future clinical perspectives. Curr. Med. Chem. 15, 2054–2070 (2008).
Fernandez, A. et al. An anticancer c-Kit kinase inhibitor is reengineered to make it more active and less cardiotoxic. J. Clin. Invest. 117, 4044–4054 (2007).
Larue, L. & Bellacosa, A. Epithelial–mesenchymal transition in development and cancer: role of phosphatidylinositol 3' kinase/Akt pathways. Oncogene 24, 7443–7454 (2005).
Apsel, B. et al. Targeted polypharmacology: discovery of dual inhibitors of tyrosine and phosphoinositide kinases. Nat. Chem. Biol. 4, 691–699 (2008).
Louvet, C. et al. Tyrosine kinase inhibitors reverse type 1 diabetes in nonobese diabetic mice. Proc. Natl Acad. Sci. USA 105, 18895–18900 (2008).
Zeisberg, M. et al. BMP-7 counteracts TGF-β1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 9, 964–968 (2003).
Zeisberg, M., Shah, A. A. & Kalluri, R. Bone morphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. J. Biol. Chem. 280, 8094–8100 (2005).
Zeisberg, M. & Kalluri, R. Reversal of experimental renal fibrosis by BMP7 provides insights into novel therapeutic strategies for chronic kidney disease. Pediatr. Nephrol. 23, 1395–1398 (2008).
Acknowledgements
The authors' studies have been supported by grants from Ministerio de Educación, Turismo y Deportes (BFU2004-00285/BFI, and SAF2007-63,893), Junta de Castilla y León (SA 001/C05), and Instituto de Salud Carlos III (RETIC RedinRen RD/0016). The authors sincerely thank Dr Angela Nieto for her critical reading of the manuscript and for her suggestions, which greatly helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Grande, M., López-Novoa, J. Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat Rev Nephrol 5, 319–328 (2009). https://doi.org/10.1038/nrneph.2009.74
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2009.74
This article is cited by
-
MiR-34a induces myofibroblast differentiation from renal fibroblasts
Clinical and Experimental Nephrology (2023)
-
IL-18 deficiency ameliorates the progression from AKI to CKD
Cell Death & Disease (2022)
-
Endothelin receptors in renal interstitial cells do not contribute to the development of fibrosis during experimental kidney disease
Pflügers Archiv - European Journal of Physiology (2021)
-
Stratified layer analysis reveals intrinsic leptin stimulates cryptal mesenchymal cells for controlling mucosal inflammation
Scientific Reports (2020)
-
Protective role of renal proximal tubular alpha-synuclein in the pathogenesis of kidney fibrosis
Nature Communications (2020)