Abstract
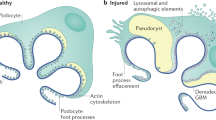
Encapsulating peritoneal sclerosis (EPS) is a severe complication of long-term peritoneal dialysis (PD) with a 50% mortality rate. EPS is characterized by progressive and excessive fibrotic thickening of the peritoneum, leading to encapsulation of the bowels and intestinal obstruction. At present, EPS cannot be detected with certainty during its early stages; however, a progressive loss of ultrafiltration capacity often precedes its development. Studies that attempted to elucidate the pathogenesis of EPS have shown that the duration of exposure to PD fluids is the most important risk factor for EPS, and that young age and possibly the effects of peritonitis are additional contributory factors. The pathophysiology of EPS is probably best described as a multiple-hit process with a central role for transforming growth factor β. A form of EPS that develops shortly after kidney transplantation has also been recognized as a distinct clinical entity, and may be a common form of EPS in countries with a high transplantation rate. Criteria have been developed to identify EPS by abdominal CT scan at the symptomatic stage, but further clinical research is needed to identify early EPS in asymptomatic patients, to clarify additional risk factors for EPS and to define optimal treatment strategies.
Key Points
-
Encapsulating peritoneal sclerosis (EPS) is a devastating syndrome of excessive fibrotic peritoneal thickening that can eventually encapsulate the bowel, leading to partial or total bowel obstruction
-
EPS occurs in 0.5–2.5% of patients on peritoneal dialysis (PD); in the majority of patients, EPS develops after PD treatment has stopped
-
The cumulative duration of exposure to PD fluids is the dominant risk factor for EPS, but young age and kidney transplantation might also be risk factors
-
The pathophysiology of EPS is probably best described as a multiple-hit process, in which expression of transforming growth factor β has a central role
-
No definitive criteria enable detection of the early stages of EPS, but patients with progressively declining ultrafiltration capacity are at risk of this condition and should be considered for hemodialysis
-
Treatment of EPS consists of maintaining good nutritional status, with corticosteroids and/or tamoxifen; surgery might be an alternative option if performed by a surgeon experienced in EPS
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kawaguchi, Y., Kawanishi, H., Mujais, S., Topley, N. & Oreopoulos, D. G. Encapsulating peritoneal sclerosis: definition, etiology, diagnosis, and treatment. International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis. Perit. Dial. Int. 20 (Suppl. 4), S43–S55 (2000).
Brown, M. C., Simpson, K., Kerssens, J. J. & Mactier, R. A. Encapsulating peritoneal sclerosis in the new millennium: a national cohort study. Clin. J. Am. Soc. Nephrol. 4, 1222–1229 (2009).
Kawanishi, H. Encapsulating peritoneal sclerosis in Japan: prospective multicenter controlled study. Perit. Dial. Int. 21 (Suppl. 3), S67–S71 (2001).
Rigby, R. J. & Hawley, C. M. Sclerosing peritonitis: the experience in Australia. Nephrol. Dial. Transplant. 13, 154–159 (1998).
Kawanishi, H. et al. Encapsulating peritoneal sclerosis in Japan: a prospective, controlled, multicenter study. Am. J. Kidney Dis. 44, 729–737 (2004).
Nomoto, Y. et al. Sclerosing encapsulating peritonitis in patients undergoing continuous ambulatory peritoneal dialysis: a report of the Japanese Sclerosing Encapsulating Peritonitis Study Group. Am. J. Kidney Dis. 28, 420–427 (1996).
Summers, A. M. et al. Single-center experience of encapsulating peritoneal sclerosis in patients on peritoneal dialysis for end-stage renal failure. Kidney Int. 68, 2381–2388 (2005).
Balasubramaniam, G. et al. The Pan-Thames EPS study: treatment and outcomes of encapsulating peritoneal sclerosis. Nephrol. Dial. Transplant. 24, 3209–3215 (2009).
Johnson, D. W. et al. Encapsulating peritoneal sclerosis: incidence, predictors, and outcomes. Kidney Int. 77, 904–912 (2010).
Korte, M. R., Boeschoten, E. W. & Betjes, M. G. The Dutch EPS Registry: increasing the knowledge of encapsulating peritoneal sclerosis. Neth. J. Med. 67, 359–362 (2009).
Summers, A. M. & Brenchley, P. E. An international encapsulating peritoneal sclerosis registry and DNA bank: why we need one now. Perit. Dial. Int. 26, 559–563 (2006).
Afthentopoulos, I. E., Passadakis, P., Oreopoulos, D. G. & Bargman, J. Sclerosing peritonitis in continuous ambulatory peritoneal dialysis patients: one center's experience and review of the literature. Adv. Ren. Replace. Ther. 5, 157–167 (1998).
Korte, M. R. et al. Increasing incidence of severe encapsulating peritoneal sclerosis after kidney transplantation. Nephrol. Dial. Transplant. 22, 2412–2414 (2007).
Garosi, G. & Oreopoulos, D. G. No need for an “expiry date” in chronic peritoneal dialysis to prevent encapsulating peritoneal sclerosis. Int. Urol. Nephrol. 41, 903–907 (2009).
Flessner, M. F. The transport barrier in intraperitoneal therapy. Am. J. Physiol. Renal Physiol. 288, F433–F442 (2005).
Williams, J. D., Craig, K. J., Topley, N. & Williams, G. T. Peritoneal dialysis: changes to the structure of the peritoneal membrane and potential for biocompatible solutions. Kidney Int. Suppl. 84, S158–S161 (2003).
Mateijsen, M. A. et al. Vascular and interstitial changes in the peritoneum of CAPD patients with peritoneal sclerosis. Perit. Dial. Int. 19, 517–525 (1999).
Williams, J. D., Craig, K. J., von Ruhland, C., Topley, N. & Williams, G. T. The natural course of peritoneal membrane biology during peritoneal dialysis. Kidney Int. Suppl. 88, S43–S49 (2003).
Honda, K., Nitta, K., Horita, S., Yumura, W. & Nihei, H. Morphological changes in the peritoneal vasculature of patients on CAPD with ultrafiltration failure. Nephron 72, 171–176 (1996).
Plum, J. et al. Peritoneal sclerosis in peritoneal dialysis patients related to dialysis settings and peritoneal transport properties. Kidney Int. Suppl. 78, S42–S47 (2001).
Nakayama, M. et al. Immunohistochemical detection of advanced glycosylation end-products in the peritoneum and its possible pathophysiological role in CAPD. Kidney Int. 51, 182–186 (1997).
Yamada, K. et al. Immunohistochemical study of human advanced glycosylation end-products (AGE) in chronic renal failure. Clin. Nephrol. 42, 354–361 (1994).
Honda, K. et al. Accumulation of advanced glycation end products in the peritoneal vasculature of continuous ambulatory peritoneal dialysis patients with low ultra-filtration. Nephrol. Dial. Transplant. 14, 1541–1549 (1999).
Kim, Y. S. et al. Advanced glycosylation end products stimulate collagen mRNA synthesis in mesangial cells mediated by protein kinase C and transforming growth factor-β. J. Lab. Clin. Med. 138, 59–68 (2001).
Schwenger, V. et al. Damage to the peritoneal membrane by glucose degradation products is mediated by the receptor for advanced glycation end-products. J. Am. Soc. Nephrol. 17, 199–207 (2006).
De Vriese, A. S., Flyvbjerg, A., Mortier, S., Tilton, R. G. & Lameire, N. H. Inhibition of the interaction of AGE-RAGE prevents hyperglycemia-induced fibrosis of the peritoneal membrane. J. Am. Soc. Nephrol. 14, 2109–2118 (2003).
Twardowski, Z. J. et al. Peritoneal equilibration test. Perit. Dial. Bull. 7, 138–147 (1987).
Park, M. S., Lee, H. A., Chu, W. S., Yang, D. H. & Hwang, S. D. Peritoneal accumulation of AGE and peritoneal membrane permeability. Perit. Dial. Int. 20, 452–460 (2000).
Smit, W., Parikova, A., Struijk, D. G. & Krediet, R. T. The difference in causes of early and late ultrafiltration failure in peritoneal dialysis. Perit. Dial. Int. 25 (Suppl. 3), S41–S45 (2005).
Garosi, G. Different aspects of peritoneal damage: fibrosis and sclerosis. Contrib. Nephrol. 163, 45–53 (2009).
Honda, K. et al. Histologic criteria for diagnosing encapsulating peritoneal sclerosis in continuous ambulatory peritoneal dialysis patients. Adv. Perit. Dial. 19, 169–175 (2003).
Garosi, G., Di Paolo, N., Sacchi, G. & Gaggiotti, E. Sclerosing peritonitis: a nosological entity. Perit. Dial. Int. 25 (Suppl. 3), S110–S112 (2005).
Sherif, A. M. et al. Comparison between the pathology of encapsulating sclerosis and simple sclerosis of the peritoneal membrane in chronic peritoneal dialysis. Ther. Apher. Dial. 12, 33–41 (2008).
Verger, C. & Celicout, B. Peritoneal permeability and encapsulating peritonitis. Lancet 1, 986–987 (1985).
Krediet, R. T. et al. The time course of peritoneal transport kinetics in continuous ambulatory peritoneal dialysis patients who develop sclerosing peritonitis. Am. J. Kidney Dis. 13, 299–307 (1989).
Mactier, R. A. The spectrum of peritoneal fibrosing syndromes in peritoneal dialysis. Adv. Perit. Dial. 16, 223–228 (2000).
Paniagua, R. et al. Correlation between peritoneal equilibration test and dialysis adequacy and transport test, for peritoneal transport type characterization. Perit. Dial. Int. 20, 53–59 (2000).
Lambie, M. L., John, B., Mushahar, L., Huckvale, C. & Davies, S. J. The peritoneal osmotic conductance is low well before the diagnosis of encapsulating peritoneal sclerosis is made. Kidney Int. 78, 611–618 (2010).
Sampimon, D. E., Coester, A. M., Struijk, D. G. & Krediet, R. T. The time course of peritoneal transport parameters in peritoneal dialysis patients who develop encapsulating peritoneal sclerosis. Nephrol. Dial. Transplant. 26, 291–298 (2010).
Sampimon, D. E., Coester, A. M., Struijk, D. G. & Krediet, R. T. Time course of peritoneal transport parameters in peritoneal dialysis patients who develop peritoneal sclerosis. Adv. Perit. Dial. 23, 107–111 (2007).
Brown, E. A. et al. Length of time on peritoneal dialysis and encapsulating peritoneal sclerosis: position paper for ISPD. Perit. Dial. Int. 29, 595–600 (2009).
Connolly, D. T. et al. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J. Clin. Invest. 84, 1470–1478 (1989).
Ha, H., Cha, M. K., Choi, H. N. & Lee, H. B. Effects of peritoneal dialysis solutions on the secretion of growth factors and extracellular matrix proteins by human peritoneal mesothelial cells. Perit. Dial. Int. 22, 171–177 (2002).
Mandl-Weber, S., Cohen, C. D., Haslinger, B., Kretzler, M. & Sitter, T. Vascular endothelial growth factor production and regulation in human peritoneal mesothelial cells. Kidney Int. 61, 570–578 (2002).
Io, H. et al. Morphologic changes of peritoneum and expression of VEGF in encapsulated peritoneal sclerosis rat models. Kidney Int. 65, 1927–1936 (2004).
Yoshio, Y. et al. TNP-470, an angiogenesis inhibitor, suppresses the progression of peritoneal fibrosis in mouse experimental model. Kidney Int. 66, 1677–1685 (2004).
Tanabe, K. et al. Endostatin peptide, an inhibitor of angiogenesis, prevents the progression of peritoneal sclerosis in a mouse experimental model. Kidney Int. 71, 227–238 (2007).
Zweers, M. M., de Waart, D. R., Smit, W., Struijk, D. G. & Krediet, R. T. Growth factors VEGF and TGF-β1 in peritoneal dialysis. J. Lab. Clin. Med. 134, 124–132 (1999).
Zweers, M. M., Struijk, D. G., Smit, W. & Krediet, R. T. Vascular endothelial growth factor in peritoneal dialysis: a longitudinal follow-up. J. Lab. Clin. Med. 137, 125–132 (2001).
Patel, P. et al. Smad3-dependent and -independent pathways are involved in peritoneal membrane injury. Kidney Int. 77, 319–328 (2010).
Bonniaud, P. et al. Smad3 null mice develop airspace enlargement and are resistant to TGF-β-mediated pulmonary fibrosis. J. Immunol. 173, 2099–2108 (2004).
Sato, M., Muragaki, Y., Saika, S., Roberts, A. B. & Ooshima, A. Targeted disruption of TGF-β1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J. Clin. Invest. 112, 1486–1494 (2003).
Border, W. A. & Noble, N. A. Transforming growth factor β in tissue fibrosis. N. Engl. J. Med. 331, 1286–1292 (1994).
Rougier, J. P., Guia, S., Hagege, J., Nguyen, G. & Ronco, P. M. PAI-1 secretion and matrix deposition in human peritoneal mesothelial cell cultures: transcriptional regulation by TGF-β1. Kidney Int. 54, 87–98 (1998).
Martin, J., Yung, S., Robson, R. L., Steadman, R. & Davies, M. Production and regulation of matrix metalloproteinases and their inhibitors by human peritoneal mesothelial cells. Perit. Dial. Int. 20, 524–533 (2000).
Hung., K. Y., Huang, J. W., Chen, C. T., Lee, P. H. & Tsai, T. J. Pentoxifylline modulates intracellular signalling of TGF-β in cultured human peritoneal mesothelial cells: implications for prevention of encapsulating peritoneal sclerosis. Nephrol. Dial. Transplant. 18, 670–676 (2003).
Hung, K. Y. et al. Dipyridamole inhibits TGF-β-induced collagen gene expression in human peritoneal mesothelial cells. Kidney Int. 60, 1249–1257 (2001).
Liu, L. et al. Prolonged peritoneal gene expression using a helper-dependent adenovirus. Perit. Dial. Int. 29, 508–516 (2009).
Margetts, P. J. et al. Transient overexpression of TGF-β1 induces epithelial mesenchymal transition in the rodent peritoneum. J. Am. Soc. Nephrol. 16, 425–436 (2005).
Margetts, P. J. et al. Gene transfer of transforming growth factor-β1 to the rat peritoneum: effects on membrane function. J. Am. Soc. Nephrol. 12, 2029–2039 (2001).
Liu, Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J. Am. Soc. Nephrol. 15, 1–12 (2004).
Jimenez-Heffernan, J. A. et al. Immunohistochemical characterization of fibroblast subpopulations in normal peritoneal tissue and in peritoneal dialysis-induced fibrosis. Virchows Arch. 444, 247–256 (2004).
Yanez-Mo, M. et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N. Engl. J. Med. 348, 403–413 (2003).
Yang, A. H., Chen, J. Y. & Lin, J. K. Myofibroblastic conversion of mesothelial cells. Kidney Int. 63, 1530–1539 (2003).
Selgas, R. et al. Epithelial-to-mesenchymal transition of the mesothelial cell--its role in the response of the peritoneum to dialysis. Nephrol. Dial. Transplant. 21 (Suppl. 2), ii2–ii7 (2006).
Shirai, K. et al. A new model of anterior subcapsular cataract: involvement of TGFβ/Smad signaling. Mol. Vis. 12, 681–691 (2006).
Banh, A. et al. Lens-specific expression of TGF-β induces anterior subcapsular cataract formation in the absence of Smad3. Invest. Ophthalmol. Vis. Sci. 47, 3450–3460 (2006).
Aroeira, L. S. et al. Mesenchymal conversion of mesothelial cells as a mechanism responsible for high solute transport rate in peritoneal dialysis: role of vascular endothelial growth factor. Am. J. Kidney Dis. 46, 938–948 (2005).
Hirahara, I. et al. The potential of matrix metalloproteinase-2 as a marker of peritoneal injury, increased solute transport, or progression to encapsulating peritoneal sclerosis during peritoneal dialysis--a multicentre study in Japan. Nephrol. Dial. Transplant. 22, 560–567 (2007).
De Vriese, A. S., Tilton, R. G., Mortier, S. & Lameire, N. H. Myofibroblast transdifferentiation of mesothelial cells is mediated by RAGE and contributes to peritoneal fibrosis in uraemia. Nephrol. Dial. Transplant. 21, 2549–2555 (2006).
Cano, A. et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2, 76–83 (2000).
Del Peso, G. et al. Epithelial-to-mesenchymal transition of mesothelial cells is an early event during peritoneal dialysis and is associated with high peritoneal transport. Kidney Int. Suppl. 108, S26–S33 (2008).
Kawanishi, H., Watanabe, H., Moriishi, M. & Tsuchiya, S. Successful surgical management of encapsulating peritoneal sclerosis. Perit. Dial. Int. 25 (Suppl. 4), S39–S47 (2005).
Honda, K. & Oda, H. Pathology of encapsulating peritoneal sclerosis. Perit. Dial. Int. 25 (Suppl. 4), S19–S29 (2005).
Augustine, T., Brown, P. W., Davies, S. D., Summers, A. M. & Wilkie, M. E. Encapsulating peritoneal sclerosis: clinical significance and implications. Nephron Clin. Pract. 111, c149–c154 (2009).
Summers, A. M., Hoff, C. M. & Topley, N. How can genetic advances impact on experimental models of encapsulating peritoneal sclerosis? Perit. Dial. Int. 28 (Suppl. 5), S16–S20 (2008).
Watson, C. J., Webb, N. J., Bottomley, M. J. & Brenchley, P. E. Identification of polymorphisms within the vascular endothelial growth factor (VEGF) gene: correlation with variation in VEGF protein production. Cytokine 12, 1232–1235 (2000).
Sampimon, D. E., Vlijm, A., Phoa, S. S., Krediet, R. T. & Struijk, D. G. Encapsulating peritoneal sclerosis in a peritoneal dialysis patient using biocompatible fluids only: is Alport syndrome a risk factor? Perit. Dial. Int. 30, 240–242 (2010).
Williams, J. D. et al. Morphologic changes in the peritoneal membrane of patients with renal disease. J. Am. Soc. Nephrol. 13, 470–479 (2002).
Korte, M. R. et al. Risk factors associated with encapsulating peritoneal sclerosis in Dutch EPS study. Perit. Dial. Int. 31, 269–278 (2011).
Habib, A. M., Preston, E. & Davenport, A. Risk factors for developing encapsulating peritoneal sclerosis in the icodextrin era of peritoneal dialysis prescription. Nephrol. Dial. Transplant. 25, 1633–1638 (2010).
Nakayama, M. et al. Risk factors and preventive measures for encapsulating peritoneal sclerosis—Jikei experience. Adv. Perit. Dial. 18, 144–148 (2002).
Moriishi, M. et al. Preservation of peritoneal catheter for prevention of encapsulating peritoneal sclerosis. Adv. Perit. Dial. 18, 149–153 (2002).
Yamamoto, T., Nagasue, K., Okuno, S. & Yamakawa, T. The role of peritoneal lavage and the prognostic significance of mesothelial cell area in preventing encapsulating peritoneal sclerosis. Perit. Dial. Int. 30, 343–352 (2010).
Linden, T., Forsback, G., Deppisch, R., Henle, T. & Wieslander, A. 3-Deoxyglucosone, a promoter of advanced glycation end products in fluids for peritoneal dialysis. Perit. Dial. Int. 18, 290–293 (1998).
Hendriks, P. M. et al. Peritoneal sclerosis in chronic peritoneal dialysis patients: analysis of clinical presentation, risk factors, and peritoneal transport kinetics. Perit. Dial. Int. 17, 136–143 (1997).
Boulanger, E. Peritoneal and systemic inflammation: the benefits of using biocompatible peritoneal dialysis fluids. Perit. Dial. Int. 28, 28–31 (2008).
Hekking, L. H. et al. Better preservation of peritoneal morphologic features and defense in rats after long-term exposure to a bicarbonate/lactate-buffered solution. J. Am. Soc. Nephrol. 12, 2775–2786 (2001).
Rippe, B. et al. Long-term clinical effects of a peritoneal dialysis fluid with less glucose degradation products. Kidney Int. 59, 348–357 (2001).
Williams, J. D. et al. The Euro-Balance Trial: the effect of a new biocompatible peritoneal dialysis fluid (balance) on the peritoneal membrane. Kidney Int. 66, 408–418 (2004).
Dombros, N. et al. European best practice guidelines for peritoneal dialysis. 5 Peritoneal dialysis solutions. Nephrol. Dial. Transplant. 20 (Suppl. 9), ix16–ix20 (2005).
Mistry, C. D., Mallick, N. P. & Gokal, R. Ultrafiltration with an isosmotic solution during long peritoneal dialysis exchanges. Lancet 2, 178–182 (1987).
Goodship, T. H. et al. Short-term studies on the use of amino acids as an osmotic agent in continuous ambulatory peritoneal dialysis. Clin. Sci. (Lond.) 73, 471–478 (1987).
Martis, L. et al. Aseptic peritonitis due to peptidoglycan contamination of pharmacopoeia standard dialysis solution. Lancet 365, 588–594 (2005).
Martikainen, T. A., Teppo, A. M., Gronhagen-Riska, C. & Ekstrand, A. V. Glucose-free dialysis solutions: inductors of inflammation or preservers of peritoneal membrane? Perit. Dial. Int. 25, 453–460 (2005).
Posthuma, N. et al. Peritoneal kinetics and mesothelial markers in CCPD using icodextrin for daytime dwell for two years. Perit. Dial. Int. 20, 174–180 (2000).
Moriishi, M. & Kawanishi, H. Fibrin degradation products are a useful marker for the risk of encapsulating peritoneal sclerosis. Adv. Perit. Dial. 24, 56–59 (2008).
Moriishi, M., Kawanishi, H. & Tsuchiya, S. Impact on peritoneal membrane of use of icodextrin-based dialysis solution in peritoneal dialysis patients. Adv. Perit. Dial. 22, 24–28 (2006).
Parikova, A., Zweers, M. M., Struijk, D. G. & Krediet, R. T. Peritoneal effluent markers of inflammation in patients treated with icodextrin-based and glucose-based dialysis solutions. Adv. Perit. Dial. 19, 186–190 (2003).
Davies, S. J. et al. Icodextrin improves the fluid status of peritoneal dialysis patients: results of a double-blind randomized controlled trial. J. Am. Soc. Nephrol. 14, 2338–2344 (2003).
Bradley, J. A. et al. Sclerosing obstructive peritonitis after continuous ambulatory peritoneal dialysis. Lancet 2, 113–114 (1983).
Yamamoto, R. et al. Risk factors for encapsulating peritoneal sclerosis in patients who have experienced peritoneal dialysis treatment. Clin. Exp. Nephrol. 9, 148–152 (2005).
Flanigan, M., Anderson, D. & Freeman, R. M. Peritoneal dialysis complicated by fungal peritonitis and peritoneal fibrosis. Am. J. Med. 76, A113–A125 (1984).
Chew, C. G., Clarkson, A. R. & Faull, R. J. Relapsing CAPD peritonitis with rapid peritoneal sclerosis due to Haemophilus influenzae. Nephrol. Dial. Transplant. 12, 821–822 (1997).
Kim, B. S. et al. Clinical characteristics of dialysis related sclerosing encapsulating peritonitis: multi-center experience in Korea. Yonsei Med. J. 46, 104–111 (2005).
Fieren, M. W., Betjes, M. G., Korte, M. R. & Boer, W. H. Posttransplant encapsulating peritoneal sclerosis: a worrying new trend? Perit. Dial. Int. 27, 619–624 (2007).
Kuriyama, S. & Tomonari, H. Corticosteroid therapy in encapsulating peritoneal sclerosis. Nephrol. Dial. Transplant. 16, 1304–1305 (2001).
Maluccio, M. et al. Tacrolimus enhances transforming growth factor-β1 expression and promotes tumor progression. Transplantation 76, 597–602 (2003).
Roberts, A. B. et al. Transforming growth factor type β: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc. Natl Acad. Sci. USA 83, 4167–4171 (1986).
van Westrhenen, R. et al. Cyclosporin A induces peritoneal fibrosis and angiogenesis during chronic peritoneal exposure to a glucose-based, lactate-buffered dialysis solution in the rat. Blood Purif. 25, 466–472 (2007).
Lin, C. H. et al. Sclerosing encapsulating peritonitis in a liver transplant patient: a case report. World J. Gastroenterol. 11, 5412–5413 (2005).
Oules, R., Challah, S. & Brunner, F. P. Case-control study to determine the cause of sclerosing peritoneal disease. Nephrol. Dial. Transplant. 3, 66–69 (1988).
Brown, P., Baddeley, H., Read, A. E., Davies, J. D. & McGarry, J. Sclerosing peritonitis, an unusual reaction to a β-adrenergic-blocking drug (practolol). Lancet 2, 1477–1481 (1974).
Holland, P. Sclerosing encapsulating peritonitis in chronic ambulatory peritoneal dialysis. Clin. Radiol. 41, 19–23 (1990).
Krestin, G. P., Kacl, G., Hoffmann, R., Keusch, G. & Burger, H. R. [The imaging diagnosis of sclerosing peritonitis (SP) following continuous ambulatory peritoneal dialysis (CAPD)]. Rofo 157, 506–511 (1992).
Campbell, S. et al. Sclerosing peritonitis: identification of diagnostic, clinical, and radiological features. Am. J. Kidney Dis. 24, 819–825 (1994).
Goodlad, C. et al. Screening for encapsulating peritoneal sclerosis in patients on peritoneal dialysis: role of CT scanning. Nephrol. Dial. Transplant. 26, 1374–1379 (2010).
Stafford-Johnson, D. B., Wilson, T. E., Francis, I. R. & Swartz, R. CT appearance of sclerosing peritonitis in patients on chronic ambulatory peritoneal dialysis. J. Comput. Assist. Tomogr. 22, 295–299 (1998).
Vlijm, A. et al. Computed tomographic findings characteristic for encapsulating peritoneal sclerosis: a case-control study. Perit. Dial. Int. 29, 517–522 (2009).
Tarzi, R. M. et al. Assessing the validity of an abdominal CT scoring system in the diagnosis of encapsulating peritoneal sclerosis. Clin. J. Am. Soc. Nephrol. 3, 1702–1710 (2008).
Lien, Y. C. et al. Clinical images: encapsulating peritoneal sclerosis. CMAJ 181, 177 (2009).
Huser, N. et al. Sclerosing encapsulating peritonitis: MRI diagnosis. Eur. Radiol. 16, 238–239 (2006).
Nakamoto, H. Encapsulating peritoneal sclerosis—a clinician's approach to diagnosis and medical treatment. Perit. Dial. Int. 25 (Suppl. 4), S30–S38 (2005).
Kawaguchi, Y. et al. Recommendations on the management of encapsulating peritoneal sclerosis in Japan, 2005: diagnosis, predictive markers, treatment, and preventive measures. Perit. Dial. Int. 25 (Suppl. 4), S83–S95 (2005).
Breborowicz, A., Breborowicz, M., Pyda, M., Polubinska, A. & Oreopoulos, D. Limitations of CA125 as an index of peritoneal mesothelial cell mass. Nephron Clin. Pract. 100, c46–c51 (2005).
Pannekeet, M. M., Koomen, G. C., Struijk, D. G. & Krediet, R. T. Dialysate CA125 in stable CAPD patients: no relation with transport parameters. Clin. Nephrol. 44, 248–254 (1995).
Do, J. Y. et al. The association between the vascular endothelial growth factor-to-cancer antigen 125 ratio in peritoneal dialysis effluent and the epithelial-to-mesenchymal transition in continuous ambulatory peritoneal dialysis. Perit. Dial. Int. 28 (Suppl. 3), S101–S106 (2008).
Sampimon, D. E. et al. Early diagnostic markers for encapsulating peritoneal sclerosis: a case-control study. Perit. Dial. Int. 30, 163–169 (2010).
Vlijm, A., de Waart, D. R., Zweers, M. M. & Krediet, R. T. Effluent hydroxyproline in experimental peritoneal dialysis. Perit. Dial. Int. 27, 210–213 (2007).
Cho, J. H. et al. Impact of systemic and local peritoneal inflammation on peritoneal solute transport rate in new peritoneal dialysis patients: a 1-year prospective study. Nephrol. Dial. Transplant. 25, 1964–1973 (2010).
Szeto, C. C. et al. Dialysate hyaluronan concentration predicts survival but not peritoneal sclerosis in continuous ambulatory peritoneal dialysis. Am. J. Kidney Dis. 36, 609–614 (2000).
de Freitas, D. et al. Nutritional management of patients undergoing surgery following diagnosis with encapsulating peritoneal sclerosis. Perit. Dial. Int. 28, 271–276 (2008).
Kawanishi, H., Moriishi, M. & Tsuchiya, S. Experience of 100 surgical cases of encapsulating peritoneal sclerosis: investigation of recurrent cases after surgery. Adv. Perit. Dial. 22, 60–64 (2006).
Kawanishi, H., Moriishi, M., Ide, K. & Dohi, K. Recommendation of the surgical option for treatment of encapsulating peritoneal sclerosis. Perit. Dial. Int. 28 (Suppl. 3), S205–S210 (2008).
Bhandari, S., Wilkinson, A. & Sellars, L. Sclerosing peritonitis: value of immunosuppression prior to surgery. Nephrol. Dial. Transplant. 9, 436–437 (1994).
Wong, C. F., Beshir, S., Khalil, A., Pai, P. & Ahmad, R. Successful treatment of encapsulating peritoneal sclerosis with azathioprine and prednisolone. Perit. Dial. Int. 25, 285–287 (2005).
Rajani, R., Smyth, J., Koffman, C. G., Abbs, I. & Goldsmith, D. J. Differential Effect of sirolimus vs prednisolone in the treatment of sclerosing encapsulating peritonitis. Nephrol. Dial. Transplant. 17, 2278–2280 (2002).
Lafrance, J. P. et al. Successful treatment of encapsulating peritoneal sclerosis with immunosuppressive therapy. Am. J. Kidney Dis. 51, e7–e10 (2008).
Junor, B. J. & McMillan, M. A. Immunosuppression in sclerosing peritonitis. Adv. Perit. Dial. 9, 187–189 (1993).
Fagugli, R. M., Selvi, A., Quintaliani, G., Bianchi, M. & Buoncristiani, U. Immunosuppressive treatment for sclerosing peritonitis. Nephrol. Dial. Transplant. 14, 1343–1345 (1999).
van Bommel, E. F., Hendriksz, T. R., Huiskes, A. W. & Zeegers, A. G. Brief communication: tamoxifen therapy for nonmalignant retroperitoneal fibrosis. Ann. Intern. Med. 144, 101–106 (2006).
Allaria, P. M., Giangrande, A., Gandini, E. & Pisoni, I. B. Continuous ambulatory peritoneal dialysis and sclerosing encapsulating peritonitis: tamoxifen as a new therapeutic agent? J. Nephrol. 12, 395–397 (1999).
Eltoum, M. A., Wright, S., Atchley, J. & Mason, J. C. Four consecutive cases of peritoneal dialysis-related encapsulating peritoneal sclerosis treated successfully with tamoxifen. Perit. Dial. Int. 26, 203–206 (2006).
Korte, M. R. et al. Tamoxifen is associated with lower mortality of encapsulating peritoneal sclerosis: results of the Dutch Multicentre EPS Study. Nephrol. Dial Transplant. 26, 691–697 (2010).
Noh, H. et al. Angiotensin II mediates high glucose-induced TGF-β1 and fibronectin upregulation in HPMC through reactive oxygen species. Perit. Dial. Int. 25, 38–47 (2005).
Kyuden, Y., Ito, T., Masaki, T., Yorioka, N. & Kohno, N. Tgf-β1 induced by high glucose is controlled by angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker on cultured human peritoneal mesothelial cells. Perit. Dial. Int. 25, 483–491 (2005).
Wolf, G. & Neilson, E. G. Angiotensin II as a renal growth factor. J. Am. Soc. Nephrol. 3, 1531–1540 (1993).
Subeq, Y. M. et al. Valsartan decreases TGF-β1 production and protects against chlorhexidine digluconate-induced liver peritoneal fibrosis in rats. Cytokine 53, 223–230 (2011).
Bozkurt, D. et al. The effects of renin-angiotensin system inhibition on regression of encapsulating peritoneal sclerosis. Perit. Dial. Int. 28 (Suppl. 5), S38–S42 (2008).
Kolesnyk, I. et al. Impact of ACE inhibitors and AII receptor blockers on peritoneal membrane transport characteristics in long-term peritoneal dialysis patients. Perit. Dial. Int. 27, 446–453 (2007).
Sampimon, D. E. et al. Use of angiotensin II inhibitors in patients that develop encapsulating peritoneal sclerosis. Perit. Dial. Int. 30, 656–659 (2010).
Zemel, D. et al. Appearance of tumor necrosis factor-α and soluble TNF-receptors I and II in peritoneal effluent of CAPD. Kidney Int. 46, 1422–1430 (1994).
Patel, P. et al. Platelet derived growth factor B and epithelial mesenchymal transition of peritoneal mesothelial cells. Matrix Biol. 29, 97–106 (2010).
Mizutani, M. et al. Connective tissue growth factor (CTGF/CCN2) is increased in peritoneal dialysis patients with high peritoneal solute transport rate. Am. J. Physiol. Renal. Physiol. 298, F721–F733 (2010).
Ogata, S., Yorioka, N. & Kohno, N. Glucose and prednisolone alter basic fibroblast growth factor expression in peritoneal mesothelial cells and fibroblasts. J. Am. Soc. Nephrol. 12, 2787–2796 (2001).
Vargha, R. et al. Ex vivo reversal of in vivo transdifferentiation in mesothelial cells grown from peritoneal dialysate effluents. Nephrol. Dial. Transplant. 21, 2943–2947 (2006).
Throckmorton, D. C., Brogden, A. P., Min, B., Rasmussen, H. & Kashgarian, M. PDGF and TGF-β mediate collagen production by mesangial cells exposed to advanced glycosylation end products. Kidney Int. 48, 111–117 (1995).
Katsutani, M., Ito, T., Masaki, T., Kohno, N. & Yorioka, N. Glucose-based PD solution, but not icodextrin-based PD solution, induces plasminogen activator inhibitor-1 and tissue-type plasminogen activator in human peritoneal mesothelial cells via ERK1/2. Ther. Apher. Dial. 11, 94–100 (2007).
Kurata, K. et al. Tissue-type plasminogen activator deficiency attenuates peritoneal fibrosis in mice. Am. J. Physiol. Renal Physiol. 297, F1510–F1517 (2009).
Collier, I. E. et al. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J. Biol. Chem. 263, 6579–6587 (1988).
Kim, J. J. et al. High glucose decreases collagenase expression and increases TIMP expression in cultured human peritoneal mesothelial cells. Nephrol. Dial. Transplant. 23, 534–541 (2008).
Hirahara, I., Ogawa, Y., Kusano, E. & Asano, Y. Activation of matrix metalloproteinase-2 causes peritoneal injury during peritoneal dialysis in rats. Nephrol. Dial. Transplant. 19, 1732–1741 (2004).
Ro, Y. et al. Inhibitory effects of matrix metalloproteinase inhibitor ONO-4817 on morphological alterations in chlorhexidine gluconate-induced peritoneal sclerosis rats. Nephrol. Dial. Transplant. 22, 2838–2848 (2007).
Alscher, D. M., Braun, N., Biegger, D. & Fritz, P. Peritoneal mast cells in peritoneal dialysis patients, particularly in encapsulating peritoneal sclerosis patients. Am. J. Kidney. Dis. 49, 452–461 (2007).
Leibovich, S. J. et al. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-α. Nature 329, 630–632 (1987).
Author information
Authors and Affiliations
Contributions
M. R. Korte and D. E. Sampimon contributed equally to all aspects of this manuscript. All authors were involved in researching data for article, discussion of content, writing, reviewing and editing of manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Korte, M., Sampimon, D., Betjes, M. et al. Encapsulating peritoneal sclerosis: the state of affairs. Nat Rev Nephrol 7, 528–538 (2011). https://doi.org/10.1038/nrneph.2011.93
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2011.93
This article is cited by
-
10-year-long survival in a PD patient with severe calcifying encapsulating peritoneal sclerosis treated with tamoxifen: a case-report
BMC Nephrology (2020)
-
Standardisierte histomorphologische Aufarbeitung von Peritonealbiopsien im Rahmen des Deutschen Peritonealbiopsieregisters (GRIP, German Registry In PD)
Der Pathologe (2020)
-
The peritoneal sieving of sodium: a simple and powerful test to rule out the onset of encapsulating peritoneal sclerosis in patients undergoing peritoneal dialysis
Journal of Nephrology (2018)
-
Severe peritoneal sclerosis after repeated pressurized intraperitoneal aerosol chemotherapy with oxaliplatin (PIPAC OX): report of two cases and literature survey
Clinical & Experimental Metastasis (2018)
-
Dissolved molecular hydrogen (H2) in Peritoneal Dialysis (PD) solutions preserves mesothelial cells and peritoneal membrane integrity
BMC Nephrology (2017)