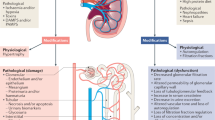
Abstract
According to guidelines published by Kidney Disease: Improving Global Outcomes, patients at risk of acute kidney injury (AKI) should be managed according to their susceptibilities and exposures. Clinical evaluation of a patient's risk of acute loss of renal function is of undisputed importance. However, such evaluations can be hindered by the complex presentations of critically ill patients and the lack of methods to detect early kidney damage. In this regard, a tool for diagnosis and stratification of patients at risk of AKI would complement clinical assessments and enable improved therapeutic decision-making. Emerging evidence suggests that 15–20% of patients who do not fulfil current serum-creatinine-based consensus criteria for AKI are nevertheless likely to have acute tubular damage, which is associated with adverse outcomes. This evidence supports reassessment of the concept and evolution of the definition of AKI to incorporate biomarkers of tubular damage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ronco, C. et al. Oliguria, creatinine and other biomarkers of acute kidney injury. Contrib. Nephrol. 164, 118–127 (2010).
Devarajan, P. Neutrophil gelatinase-associated lipocalin: a troponin-like biomarker for human acute kidney injury. Nephrology (Carlton) 15, 419–428 (2010).
Vives, M. et al. External validation and comparison of three scores to predict renal replacement therapy after cardiac surgery: a multicenter cohort. Int. J. Artif. Organs 34, 329–338 (2011).
Heise, D., Sundermann, D., Braeuer, A. & Quintel, M. Validation of a clinical score to determine the risk of acute renal failure after cardiac surgery. Eur. J. Cardiothorac. Surg. 37, 710–716 (2010).
Balasubramanian, G. et al. Early nephrologist involvement in hospital-acquired acute kidney injury: a pilot study. Am. J. Kidney Dis. 57, 228–234 (2011).
Colpaert, K. et al. Impact of real-time electronic alerting of acute kidney injury on therapeutic intervention and progression of RIFLE class. Crit. Care Med. 40, 1164–1170 (2012).
Haase, M. et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J. Am. Coll. Cardiol. 57, 1752–1761 (2011).
Srisawat, N. et al. Plasma neutrophil gelatinase-associated lipocalin predicts recovery from acute kidney injury following community-acquired pneumonia. Kidney Int. 80, 545–552 (2011).
Krawczeski, C. D. et al. Neutrophil gelatinase-associated lipocalin concentrations predict development of acute kidney injury in neonates and children after cardiopulmonary bypass. J. Pediatr. 158, 1009–1015 (2011).
de Geus, H. R., Bakker, J., Lesaffre, E. M. & le Noble, J. L. Neutrophil gelatinase-associated lipocalin at ICU admission predicts acute kidney injury in adult patients. Am. J. Respir. Crit. Care Med. 183, 907–914 (2011).
Nickolas, T. L. et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J. Am. Coll. Cardiol. 59, 246–255 (2012).
Portilla, D. et al. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 73, 465–472 (2008).
Wronski, T. et al. The step response: a method to characterize mechanisms of renal blood flow autoregulation. Am. J. Physiol. Renal Physiol. 285, F758–F764 (2003).
Mishra, J. et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J. Am. Soc. Nephrol. 14, 2534–2543 (2003).
KDIGO Section 2: AKI definition. Kidney Int. Suppl. 2, 7–24 (2012).
KDIGO Clinical Practice Guideline for Acute Kidney Injury. Section 3: prevention and treatment of AKI. Kidney Int. Suppl. 2, 37–68 (2012).
Cruz, D. N., Ricci, Z. & Ronco, C. Clinical review: RIFLE and AKIN—time for reappraisal. Crit. Care 13, 211 (2009).
[No authors listed] Diagnosis of acute kidney injury using functional and damage biomarkers: workgroup statements from the 10th Acute Dialysis Quality Initiative consensus conference. Clin. J. Am. Soc. Nephrol. (in press).
Uchino, S. et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294, 813–818 (2005).
Thakar, C. V., Arrigain, S., Worley, S., Yared, J. P. & Paganini, E. P. A clinical score to predict acute renal failure after cardiac surgery. J. Am. Soc. Nephrol. 16, 162–168 (2005).
Vincent, J. L. et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 22, 707–710 (1996).
Nejat, M. et al. Urinary cystatin C is diagnostic of acute kidney injury and sepsis, and predicts mortality in the intensive care unit. Crit. Care 14, R85 (2010).
Paragas, N. et al. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat. Med. 17, 216–222 (2011).
Haase, M. et al. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am. J. Kidney Dis. 54, 1012–1024 (2009).
Krawczeski, C. D. et al. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J. Am. Coll. Cardiol. 58, 2301–2309 (2011).
Schmidt-Ott, K. et al. Can urinary kidney injury biomarkers affect clinical decision making in the emergency department? Poster presented at 22nd American Society of Nephrology Kidney Week (2011).
Haase M. et al. Sodium bicarbonate to prevent increases in serum creatinine after cardiac surgery: a pilot double-blind, randomized controlled trial. Crit. Care Med. 37, 39–47 (2009).
Sharfuddin, A. A. & Molitoris, B. A. Pathophysiology of ischemic acute kidney injury. Nat. Rev. Nephrol. 7, 189–200 (2011).
Grgic, I. et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. http://dx.doi.org/10.1038/ki.2012.20.
Jones, J. et al. Association of complete recovery from acute kidney injury with incident CKD stage 3 and all-cause mortality. Am. J. Kidney Dis. 60, 402–408 (2012).
Coca, S. G., Singanamala, S. & Parikh, C. R. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 81, 442–448 (2012).
Ronco, C. & Rosner, M. H. Acute kidney injury and residual renal function. Crit. Care 16, 144 (2012).
Lai, C. F. et al. Kidney function decline after a non-dialysis-requiring acute kidney injury is associated with higher long-term mortality in critically ill survivors. Crit. Care 16, R123 (2012).
Haase, M., Bellomo, R. & Haase-Fielitz, A. Novel biomarkers, oxidative stress, and the role of labile iron toxicity in cardiopulmonary bypass-associated acute kidney injury. J. Am. Coll. Cardiol. 55, 2024–2033 (2010).
Peng, Z. Y. et al. Bactericidal antibiotics temporarily increase inflammation and worsen acute kidney injury in experimental sepsis. Crit. Care Med. 40, 538–543 (2012).
Ko, G. J. et al. Transcriptional analysis of kidney tissue during recovery from AKI reveals possible roles for NGAL and KIM-1 as biomarkers of AKI-to-CKD transition. Am. J. Physiol. Renal Physiol. 298, F1472–F1483 (2010).
Mori, K. et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia–reperfusion injury. J. Clin. Invest. 115, 610–621 (2005).
Endre, Z. H. et al. Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney Int. 79, 1119–1130 (2011).
Author information
Authors and Affiliations
Contributions
All authors contributed to researching the data for the article, discussions of its content, writing the article and review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M. Haase has received grants from Astute Medical and lecture fees from Abbott, Alere, Fresenius and Roche. J. A. Kellum has received grants from Alere, Astute Medical and Gambro and Baxter, and has acted as a consultant for Abbott, Alere, Astute Medical, Fresenius and Gambro and Baxter. C. Ronco has received speaker honoraria from Abbott, Alere, Gambro, General Electric Company and Merck, and has acted as a consultant for Asahi Kasei Medical and CardioBridge.
Rights and permissions
About this article
Cite this article
Haase, M., Kellum, J. & Ronco, C. Subclinical AKI—an emerging syndrome with important consequences. Nat Rev Nephrol 8, 735–739 (2012). https://doi.org/10.1038/nrneph.2012.197
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2012.197
This article is cited by
-
Use of urine neutrophil gelatinase-associated lipocalin for nephrotoxic medication acute kidney injury screening in neonates
Journal of Perinatology (2024)
-
Evaluation of risk stratification for acute kidney injury: a comparative analysis of EKFC, 2009 and 2021 CKD-EPI glomerular filtration estimating equations
Journal of Nephrology (2024)
-
Pre-clinical evaluation of biomarkers for the early detection of nephrotoxicity following alpha-particle radioligand therapy
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
Acute kidney disease beyond day 7 after major surgery: a secondary analysis of the EPIS-AKI trial
Intensive Care Medicine (2024)
-
The outcome of acute kidney injury substages based on urinary cystatin C in critically ill children
Annals of Intensive Care (2023)