Key Points
-
Muscle atrophy frequently complicates the course of chronic kidney disease (CKD) and is associated with excess morbidity and mortality
-
CKD-induced mechanisms that cause muscle atrophy include activation of the ubiquitin–proteasome system, caspase-3 and myostatin
-
Triggers that activate proteolysis in CKD include metabolic acidosis, defects in insulin/insulin-like growth factor 1 (IGF-1) intracellular signalling, inflammation and catabolic responses to microRNAs
-
Current strategies aimed at preventing muscle atrophy include correction of acidosis plus exercise; specific therapies that inhibit myostatin or signal transducer and activator of transcription 3 (Stat3) are being developed
Abstract
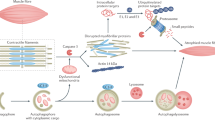
In patients with chronic kidney disease (CKD), loss of cellular proteins increases the risks of morbidity and mortality. Persistence of muscle protein catabolism in CKD results in striking losses of muscle proteins as whole-body protein turnover is great; even small but persistent imbalances between protein synthesis and degradation cause substantial protein loss. No reliable methods to prevent CKD-induced muscle wasting currently exist, but mechanisms that control cellular protein turnover have been identified, suggesting that therapeutic strategies will be developed to suppress or block protein loss. Catabolic pathways that cause protein wasting include activation of the ubiquitin–proteasome system (UPS), caspase-3, lysosomes and myostatin (a negative regulator of skeletal muscle growth). These pathways can be initiated by complications associated with CKD, such as metabolic acidosis, defective insulin signalling, inflammation, increased angiotensin II levels, abnormal appetite regulation and impaired microRNA responses. Inflammation stimulates cellular signalling pathways that activate myostatin, which accelerates UPS-mediated catabolism. Blocking this pathway can prevent loss of muscle proteins. Myostatin inhibition could yield new therapeutic directions for blocking muscle protein wasting in CKD or disorders associated with its complications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Griffiths, R. D. Muscle mass, survival, and the elderly ICU patient. Nutrition 12, 456–458 (1996).
Windsor, J. A. & Hill, G. L. Risk factors for postoperative pneumonia. The importance of protein depletion. Ann. Surg. 208, 209–214 (1988).
Gracia-Iguacel, C. et al. Prevalence of protein-energy wasting syndrome and its association with mortality in haemodialysis patients in a centre in Spain. Nefrologia 33, 495–505 (2013).
Go, A. S., Chertow, G. M., Fan, D., McCulloch, C. E. & Hsu, C. Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351, 1296–1305 (2004).
United States Renal Data System. USRDS 2009 Annual Data Report: Atlas of End-Stage Renal Disease in the United States [online], (2009).
Lowrie, E. G. & Lew, N. L. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am. J. Kidney Dis. 15, 458–482 (1990).
Stenvinkel, P., Heimbürger, O. & Lindholm, B. Wasting, but not malnutrition, predicts cardiovascular mortality in end-stage renal disease. Nephrol. Dial. Transplant. 19, 2181–2183 (2004).
Carrero, J. J. et al. Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin. Nutr. 27, 557–564 (2008).
Hakim, R. M. & Lazarus, J. M. Initiation of dialysis. J. Am. Soc. Nephrol. 6, 1319–1328 (1995).
Mitch, W. E. Malnutrition: a frequent misdiagnosis for hemodialysis patients. J. Clin. Invest. 110, 437–439 (2002).
Fouque, D. et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 73, 391–398 (2008).
Abrass, C. K. Overview: obesity: what does it have to do with kidney disease? J. Am. Soc. Nephrol. 15, 2768–2772 (2004).
Ikizler, T. A. et al. Hemodialysis stimulates muscle and whole body protein loss and alters substrate oxidation. Am. J. Physiol. Endocrinol. Metab. 282, E107–E116 (2002).
Pupim, L. B. et al. Intradialytic parenteral nutrition improves protein and energy homeostasis in chronic hemodialysis patients. J. Clin. Invest. 110, 483–492 (2002).
Pupim, L. B., Majchrzak, K. M., Flakoll, P. J. & Ikizler, T. A. Intradialytic oral nutrition improves protein homeostasis in chronic hemodialysis patients with deranged nutritional status. J. Am. Soc. Nephrol. 17, 3149–3157 (2006).
Carrero, J. J. et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 23, 77–90 (2013).
Cano, N. J. et al. Intradialytic parenteral nutrition does not improve survival in malnourished hemodialysis patients: a 2-year multicenter, prospective, randomized study. J. Am. Soc. Nephrol. 18, 2583–2591 (2007).
Dobre, M., Meyer, T. W. & Hostetter, T. H. Searching for uremic toxins. Clin. J. Am. Soc. Nephrol. 8, 322–327 (2013).
Mitch, W. E. & Ikizler, T. A. (Eds) Handbook of Nutrition and the Kidney (Lippincott Williams & Wilkins, 2010).
Tripathy, K., Klahr, S. & Lotero, H. Utilization of exogenous urea nitrogen in malnourished adults. Metabolism 19, 253–262 (1970).
Kaysen, G. A. et al. Inflammation and reduced albumin synthesis associated with stable decline in serum albumin in hemodialysis patients. Kidney Int. 65, 1408–1415 (2004).
Smith, G., Robinson, P. H. & Fleck, A. Serum albumin distribution in early treated anorexia nervosa. Nutrition 12, 677–684 (1996).
Mitch, W. E. & Goldberg, A. L. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N. Engl. J. Med. 335, 1897–1905 (1996).
Zhang, L., Wang, X. H., Wang, H., Du, J. & Mitch, W. E. Satellite cell dysfunction and impaired IGF-1 signaling cause CKD-induced muscle atrophy. J. Am. Soc. Nephrol. 21, 419–427 (2010).
Grefte, S., Kuijpers-Jagtman, A. M., Torensma, R. & Von den Hoff, J. W. Skeletal muscle development and regeneration. Stem Cells Dev. 16, 857–868 (2007).
Ding, H., Gao, X. L., Hirschberg, R., Vadgama, J. V. & Kopple, J. D. Impaired actions of insulin-like growth factor 1 on protein synthesis and degradation in skeletal muscle of rats with chronic renal failure. Evidence for a postreceptor defect. J. Clin. Invest. 97, 1064–1075 (1996).
Castellino, P. et al. Glucose and amino acid metabolism in chronic renal failure: effect of insulin and amino acids. Am. J. Physiol. 262, F168–F176 (1992).
Adey, D., Kumar, R., McCarthy, J. T. & Nair, K. S. Reduced synthesis of muscle proteins in chronic renal failure. Am. J. Physiol. Endocrinol. Metab. 278, E219–E225 (2000).
Bohé, J., Low, A., Wolfe, R. R. & Rennie, M. J. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J. Physiol. 552, 315–324 (2003).
May, R. C., Kelly, R. A. & Mitch, W. E. Mechanisms for defects in muscle protein metabolism in rats with chronic uremia. Influence of metabolic acidosis. J. Clin. Invest. 79, 1099–1103 (1987).
Wang, X. H., Du, J., Klein, J. D., Bailey, J. L. & Mitch, W. E. Exercise ameliorates chronic kidney disease-induced defects in muscle protein metabolism and progenitor cell function. Kidney Int. 76, 751–759 (2009).
Bailey, J. L. et al. The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway. J. Clin. Invest. 97, 1447–1453 (1996).
Bailey, J. L., Zheng, B., Hu, Z., Price, S. R. & Mitch, W. E. Chronic kidney disease causes defects in signaling through the insulin receptor substrate/phosphatidylinositol 3-kinase/Akt pathway: implications for muscle atrophy. J. Am. Soc. Nephrol. 17, 1388–1394 (2006).
Raj, D. S. et al. Protein turnover and amino acid transport kinetics in end-stage renal disease. Am. J. Physiol. Endocrinol. Metab. 286, E136–E143 (2004).
Reaich, D. et al. Correction of acidosis in humans with CRF decreases protein degradation and amino acid oxidation. Am. J. Physiol. 265, E230–E235 (1993).
Ballmer, P. E. et al. Chronic metabolic acidosis decreases albumin synthesis and induces negative nitrogen balance in humans. J. Clin. Invest. 95, 39–45 (1995).
Lecker, S. H., Goldberg, A. L. & Mitch, W. E. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J. Am. Soc. Nephrol. 17, 1807–1819 (2006).
Rock, K. L. et al. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 78, 761–771 (1994).
Lecker, S. H. & Mitch, W. E. Proteolysis by the ubiquitin-proteasome system and kidney disease. J. Am. Soc. Nephrol. 22, 821–824 (2011).
Wang, X. H. & Mitch, W. E. Muscle wasting from kidney failure—a model for catabolic conditions. Int. J. Biochem. Cell Biol. 45, 2230–2238 (2013).
Saric, T., Graef, C. I. & Goldberg, A. L. Pathway for degradation of peptides generated by proteasomes: a key role for thimet oligopeptidase and other metallopeptidases. J. Biol. Chem. 279, 46723–46732 (2004).
Groll, M. et al. A gated channel into the proteasome core particle. Nat. Struct. Biol. 7, 1062–1067 (2000).
Du, J. et al. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J. Clin. Invest. 113, 115–123 (2004).
Workeneh, B. T. et al. Development of a diagnostic method for detecting increased muscle protein degradation in patients with catabolic conditions. J. Am. Soc. Nephrol. 17, 3233–3239 (2006).
Wang, X. H. et al. Caspase-3 cleaves specific 19 S proteasome subunits in skeletal muscle stimulating proteasome activity. J. Biol. Chem. 285, 21249–21257 (2010).
Lecker, S. H. et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 18, 39–51 (2004).
Mizushima, N. & Komatsu, M. Autophagy: renovation of cells and tissues. Cell 147, 728–741 (2011).
Sandri, M. Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. Int. J. Biochem. Cell Biol. 45, 2121–2129 (2013).
Mitch, W. E. et al. Metabolic acidosis stimulates muscle protein degradation by activating the adenosine triphosphate-dependent pathway involving ubiquitin and proteasomes. J. Clin. Invest. 93, 2127–2133 (1994).
Price, S. R. et al. Muscle wasting in insulinopenic rats results from activation of the ATP-dependent, ubiquitin-proteasome proteolytic pathway by a mechanism including gene transcription. J. Clin. Invest. 98, 1703–1708 (1996).
Hu, Z., Wang, H., Lee, I. H., Du, J. & Mitch, W. E. Endogenous glucocorticoids and impaired insulin signaling are both required to stimulate muscle wasting under pathophysiological conditions in mice. J. Clin. Invest. 119, 3059–3069 (2009).
Han, H. Q., Zhou, X., Mitch, W. E. & Goldberg, A. L. Myostatin/activin pathway antagonism: molecular basis and therapeutic potential. Int. J. Biochem. Cell Biol. 45, 2333–2347 (2013).
Sartori, R. et al. Smad2 and 3 transcription factors control muscle mass in adulthood. Am. J. Physiol. Cell Physiol. 296, C1248–C1257 (2009).
McPherron, A. C., Lawler, A. M. & Lee, S. J. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 387, 83–90 (1997).
Zhang, L. et al. Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. FASEB J. 25, 1653–1663 (2011).
Lee, S. W. et al. Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J. Am. Soc. Nephrol. 15, 1537–1545 (2004).
Chanutin, A. & Ludewig, S. Experimental renal insufficiency produced by partial nephrectomy. V. Diets containing whole dried meat. Arch. Intern. Med. 58, 60–80 (1936).
May, R. C., Kelly, R. A. & Mitch, W. E. Metabolic acidosis stimulates protein degradation in rat muscle by a glucocorticoid-dependent mechanism. J. Clin. Invest. 77, 614–621 (1986).
de Brito-Ashurst, I., Varagunam, M., Raftery, M. J. & Yaqoob, M. M. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J. Am. Soc. Nephrol. 20, 2075–2084 (2009).
Isozaki, U., Mitch, W. E., England, B. K. & Price, S. R. Protein degradation and increased mRNAs encoding proteins of the ubiquitin-proteasome proteolytic pathway in BC3H1 myocytes require an interaction between glucocorticoids and acidification. Proc. Natl Acad. Sci. USA 93, 1967–1971 (1996).
Price, S. R., England, B. K., Bailey, J. L., Van Vreede, K. & Mitch, W. E. Acidosis and glucocorticoids concomitantly increase ubiquitin and proteasome subunit mRNAs in rat muscle. Am. J. Physiol. 267, C955–C960 (1994).
Bailey, J. L., England, B. K., Long, R. C. Jr, Weissman, J. & Mitch, W. E. Experimental acidemia and muscle cell pH in chronic acidosis and renal failure. Am. J. Physiol. 269, C706–C712 (1995).
Folli, F., Saad, M. J. & Kahn, C. R. Insulin receptor/IRS-1/PI 3-kinase signaling system in corticosteroid-induced insulin resistance. Acta Diabetol. 33, 185–192 (1996).
DeFronzo, R. A. & Beckles, A. D. Glucose intolerance following chronic metabolic acidosis in man. Am. J. Physiol. 236, E328–E334 (1979).
Wang, X., Hu, Z., Hu, J., Du, J. & Mitch, W. E. Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 147, 4160–4168 (2006).
Zhou, Q., Du, J., Hu, Z., Walsh, K. & Wang, X. H. Evidence for adipose-muscle cross talk: opposing regulation of muscle proteolysis by adiponectin and fatty acids. Endocrinology 148, 5696–5705 (2007).
Franke, T. F., Hornik, C. P., Segev, L., Shostak, G. A. & Sugimoto, C. PI3K/Akt and apoptosis: size matters. Oncogene 22, 8983–8998 (2003).
Katso, R. et al. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 17, 615–675 (2001).
Musarò, A. et al. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat. Genet. 27, 195–200 (2001).
Datta, S. R., Brunet, A. & Greenberg, M. E. Cellular survival: a play in three Akts. Genes Dev. 13, 2905–2927 (1999).
Sandri, M. et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117, 399–412 (2004).
Hu, Z. et al. PTEN inhibition improves muscle regeneration in mice fed a high-fat diet. Diabetes 59, 1312–1320 (2010).
Maehama, T. & Dixon, J. E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273, 13375–13378 (1998).
Dupont, J., Renou, J. P., Shani, M., Hennighausen, L. & LeRoith, D. PTEN overexpression suppresses proliferation and differentiation and enhances apoptosis of the mouse mammary epithelium. J. Clin. Invest. 110, 815–825 (2002).
Hu, Z. et al. PTEN expression contributes to the regulation of muscle protein degradation in diabetes. Diabetes 56, 2449–2456 (2007).
Thomas, S. S., Dong, Y., Zhang, L. & Mitch, W. E. Signal regulatory protein-α interacts with the insulin receptor contributing to muscle wasting in chronic kidney disease. Kidney Int. 84, 308–316 (2013).
Cheung, W. W., Paik, K. H. & Mak, R. H. Inflammation and cachexia in chronic kidney disease. Pediatr. Nephrol. 25, 711–724 (2010).
Zhang, L. et al. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J. Am. Soc. Nephrol. 20, 604–612 (2009).
Menon, V. et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 68, 766–772 (2005).
Knight, E. L. et al. Kidney dysfunction, inflammation, and coronary events: a prospective study. J. Am. Soc. Nephrol. 15, 1897–1903 (2004).
Hung, A. M., Ellis, C. D., Shintani, A., Booker, C. & Ikizler, T. A. IL-1β receptor antagonist reduces inflammation in hemodialysis patients. J. Am. Soc. Nephrol. 22, 437–442 (2011).
Song, Y. H. et al. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J. Clin. Invest. 115, 451–458 (2005).
Rui, L., Yuan, M., Frantz, D., Shoelson, S. & White, M. F. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J. Biol. Chem. 277, 42394–42398 (2002).
Zhang, L. et al. Chemokine CXCL16 regulates neutrophil and macrophage infiltration into injured muscle, promoting muscle regeneration. Am. J. Pathol. 175, 2518–2527 (2009).
Zhang, L. et al. Stat3 activation links a C/EBPδ to myostatin pathway to stimulate loss of muscle mass. Cell Metab. 18, 368–379 (2013).
Cheung, W. et al. Role of leptin and melanocortin signaling in uremia-associated cachexia. J. Clin. Invest. 115, 1659–1665 (2005).
Cheung, W. W. et al. Peripheral administration of the melanocortin-4 receptor antagonist NBI-12i ameliorates uremia-associated cachexia in mice. J. Am. Soc. Nephrol. 18, 2517–2524 (2007).
Cheung, W. W., Rosengren, S., Boyle, D. L. & Mak, R. H. Modulation of melanocortin signaling ameliorates uremic cachexia. Kidney Int. 74, 180–186 (2008).
Cheung, W. W. & Mak, R. H. Melanocortin antagonism ameliorates muscle wasting and inflammation in chronic kidney disease. Am. J. Physiol. Renal Physiol. 303, F1315–F1324 (2012).
Wang, X. H. et al. Decreased miR-29 suppresses myogenesis in CKD. J. Am. Soc. Nephrol. 22, 2068–2076 (2011).
Wada, S. et al. Translational suppression of atrophic regulators by microRNA-23a integrates resistance to skeletal muscle atrophy. J. Biol. Chem. 286, 38456–38465 (2011).
Lin, Z. et al. miR-23a functions downstream of NFATc3 to regulate cardiac hypertrophy. Proc. Natl Acad. Sci. USA 106, 12103–12108 (2009).
Xu, J. et al. Transcription factor FoxO1, the dominant mediator of muscle wasting in chronic kidney disease, is inhibited by microRNA-486. Kidney Int. 82, 401–411 (2012).
Small, E. M. et al. Regulation of PI3-kinase/Akt signaling by muscle-enriched microRNA-486. Proc. Natl Acad. Sci. USA 107, 4218–4223 (2010).
Dey, B. K., Gagan, J. & Dutta, A. miR-206 and -486 induce myoblast differentiation by downregulating Pax7. Mol. Cell. Biol. 31, 203–214 (2011).
Alexander, M. S. et al. Regulation of DMD pathology by an ankyrin-encoded miRNA. Skelet. Muscle 1, 27 (2011).
Elia, L. et al. Reciprocal regulation of microRNA-1 and insulin-like growth factor-1 signal transduction cascade in cardiac and skeletal muscle in physiological and pathological conditions. Circulation 120, 2377–2385 (2009).
Huang, M. B., Xu, H., Xie, S. J., Zhou, H. & Qu, L. H. Insulin-like growth factor-1 receptor is regulated by microRNA-133 during skeletal myogenesis. PLoS One 6, e29173 (2011).
Yan, B., Zhu, C. D., Guo, J. T., Zhao, L. H. & Zhao, J. L. miR-206 regulates the growth of the teleost tilapia (Oreochromis niloticus) through the modulation of IGF-1 gene expression. J. Exp. Biol. 216, 1265–1269 (2013).
Huang, Z., Chen, X., Yu, B., He, J. & Chen, D. MicroRNA-27a promotes myoblast proliferation by targeting myostatin. Biochem. Biophys. Res. Commun. 423, 265–269 (2012).
Allen, D. L. & Loh, A. S. Posttranscriptional mechanisms involving microRNA-27a and b contribute to fast-specific and glucocorticoid-mediated myostatin expression in skeletal muscle. Am. J. Physiol. Cell Physiol. 300, C124–C137 (2011).
Chen, X., Huang, Z., Chen, D., Yang, T. & Liu, G. MicroRNA-27a is induced by leucine and contributes to leucine-induced proliferation promotion in C2C12 cells. Int. J. Mol. Sci. 14, 14076–14084 (2013).
Fouque, D. & Mitch, W. E. in Brenner and Rector's The Kidney 9th edn Ch. 60 (Eds Taal, M. W. et al.) 2170–2204 (Elsevier, 2012).
Moore, L. W. et al. The mean dietary protein intake at different stages of chronic kidney disease is higher than current guidelines. Kidney Int. 83, 724–732 (2013).
Chen, Y., Sood, S., Biada, J., Roth, R. & Rabkin, R. Increased workload fully activates the blunted IRS-1/PI3-kinase/Akt signaling pathway in atrophied uremic muscle. Kidney Int. 73, 848–855 (2008).
Sun, D. F., Chen, Y. & Rabkin, R. Work-induced changes in skeletal muscle IGF-1 and myostatin gene expression in uremia. Kidney Int. 70, 453–459 (2006).
Majchrzak, K. M., Pupim, L. B., Flakoll, P. J. & Ikizler, T. A. Resistance exercise augments the acute anabolic effects of intradialytic oral nutritional supplementation. Nephrol. Dial. Transplant. 23, 1362–1369 (2008).
Kopple, J. D. et al. Exercise in maintenance hemodialysis patients induces transcriptional changes in genes favoring anabolic muscle. J. Am. Soc. Nephrol. 18, 2975–2986 (2007).
Onda, A. et al. Acupuncture ameliorated skeletal muscle atrophy induced by hindlimb suspension in mice. Biochem. Biophys. Res. Commun. 410, 434–439 (2011).
Takaoka, Y. et al. Electroacupuncture suppresses myostatin gene expression: cell proliferative reaction in mouse skeletal muscle. Physiol. Genomics 30, 102–110 (2007).
Hu, L. et al. Low-frequency electrical stimulation attenuates muscle atrophy in CKD—a potential treatment strategy. J. Am. Soc. Nephrol. (in press).
Kobayashi, S., Maesato, K., Moriya, H., Ohtake, T. & Ikeda, T. Insulin resistance in patients with chronic kidney disease. Am. J. Kidney Dis. 45, 275–280 (2005).
Wagner, K. R. et al. A phase I/II trial of MYO-029 in adult subjects with muscular dystrophy. Ann. Neurol. 63, 561–571 (2008).
Zhou, X. et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 142, 531–543 (2010).
Redell, M. S., Ruiz, M. J., Alonzo, T. A., Gerbing, R. B. & Tweardy, D. J. Stat3 signaling in acute myeloid leukemia: ligand-dependent and -independent activation and induction of apoptosis by a novel small-molecule Stat3 inhibitor. Blood 117, 5701–5709 (2011).
Macdonald, J. H. et al. Nandrolone decanoate as anabolic therapy in chronic kidney disease: a randomized phase II dose-finding study. Nephron Clin. Pract. 106, c125–c135 (2007).
Butterfield, G. E. et al. Effect of rhGH and rhIGF-I treatment on protein utilization in elderly women. Am. J. Physiol. 272, E94–E99 (1997).
Bonaldo, P. & Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech. 6, 25–39 (2013).
Garibotto, G. et al. Effects of recombinant human growth hormone on muscle protein turnover in malnourished hemodialysis patients. J. Clin. Invest. 99, 97–105 (1997).
Sun, D. F. et al. Chronic uremia attenuates growth hormone-induced signal transduction in skeletal muscle. J. Am. Soc. Nephrol. 15, 2630–2636 (2004).
Mehls, O., Ritz, E., Hunziker, E. B., Tönshoff, B. & Heinrich, U. Role of growth hormone in growth failure of uraemia—perspectives for application of recombinant growth hormone. Acta Paediatr. Scand. Suppl. 343, 118–126 (1988).
Cohn, R. D. et al. Angiotensin II type 1 receptor blockade attenuates TGF-β-induced failure of muscle regeneration in multiple myopathic states. Nat. Med. 13, 204–210 (2007).
Burks, T. N. et al. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci. Transl. Med. 3, 82ra37 (2011).
Barazzoni, R., Gortan Cappellari, G., Zanetti, M. & Guarnieri, G. Ghrelin and muscle metabolism in chronic uremia. J. Ren. Nutr. 22, 171–175 (2012).
Porporato, P. E. et al. Acylated and unacylated ghrelin impair skeletal muscle atrophy in mice. J. Clin. Invest. 123, 611–622 (2013).
Wynne, K. et al. Subcutaneous ghrelin enhances acute food intake in malnourished patients who receive maintenance peritoneal dialysis: a randomized, placebo-controlled trial. J. Am. Soc. Nephrol. 16, 2111–2118 (2005).
Cheung, W. W. et al. A pegylated leptin antagonist ameliorates CKD-associated cachexia in mice. J. Am. Soc. Nephrol. 25, 119–128 (2014).
Caron, A. Z. et al. The proteasome inhibitor MG132 reduces immobilization-induced skeletal muscle atrophy in mice. BMC Musculoskelet. Disord. 12, 185 (2011).
Jamart, C., Raymackers, J. M., Li An, G., Deldicque, L. & Francaux, M. Prevention of muscle disuse atrophy by MG132 proteasome inhibitor. Muscle Nerve 43, 708–716 (2011).
Enrico, O. et al. Unexpected cardiotoxicity in haematological bortezomib treated patients. Br. J. Haematol. 138, 396–397 (2007).
Eddins, M. J. et al. Targeting the ubiquitin E3 ligase MuRF1 to inhibit muscle atrophy. Cell Biochem. Biophys. 60, 113–118 (2011).
Naguibneva, I. et al. The microRNA miR-181 targets the homeobox protein Hox-A11 during mammalian myoblast differentiation. Nat. Cell Biol. 8, 278–284 (2006).
Russell, A. P. et al. Disruption of skeletal muscle mitochondrial network genes and miRNAs in amyotrophic lateral sclerosis. Neurobiol. Dis. 49C, 107–117 (2012).
Wang, L. et al. MiR-23a inhibits myogenic differentiation through down regulation of fast myosin heavy chain isoforms. Exp. Cell Res. 318, 2324–2334 (2012).
Wang, H. et al. NF-κB-YY1-miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer Cell 14, 369–381 (2008).
Winbanks, C. E. et al. TGF-β regulates miR-206 and miR-29 to control myogenic differentiation through regulation of HDAC4. J. Biol. Chem. 286, 13805–13814 (2011).
Mersey, B. D., Jin, P. & Danner, D. J. Human microRNA (miR29b) expression controls the amount of branched chain α-ketoacid dehydrogenase complex in a cell. Hum. Mol. Genet. 14, 3371–3377 (2005).
Acknowledgements
This work was supported by NIAMS R01 AR060268 (X.H.W.) and NIDDK R37 DK037175 (W.E.M.).
Author information
Authors and Affiliations
Contributions
Both authors researched the data for the article, provided substantial contributions to discussions of its content, wrote the article and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wang, X., Mitch, W. Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol 10, 504–516 (2014). https://doi.org/10.1038/nrneph.2014.112
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2014.112
This article is cited by
-
The phase angle cut-off point capable of discriminating hemodialysis patients with reduced exercise tolerance: a cross-sectional study
BMC Sports Science, Medicine and Rehabilitation (2024)
-
Muscle ultrasound to diagnose sarcopenia in chronic kidney disease: a systematic review and bayesian bivariate meta-analysis
BMC Nephrology (2024)
-
Advances in pediatric acute kidney injury pharmacology and nutrition: a report from the 26th Acute Disease Quality Initiative (ADQI) consensus conference
Pediatric Nephrology (2024)
-
Mechanism for exercise-mediated prevention against muscle wasting on extensor digitorum longus muscle in Spontaneously Diabetic Torii fatty rats
The Journal of Physiological Sciences (2023)
-
Exercise and chronic kidney disease: potential mechanisms underlying the physiological benefits
Nature Reviews Nephrology (2023)