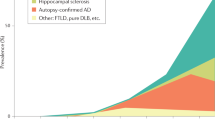
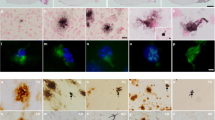
Abstract
Despite tremendous investments in understanding the complex molecular mechanisms underlying Alzheimer disease (AD), recent clinical trials have failed to show efficacy. A potential problem underlying these failures is the assumption that the molecular mechanism mediating the genetically determined form of the disease is identical to the one resulting in late-onset AD. Here, we integrate experimental evidence outside the 'spotlight' of the genetic drivers of amyloid-β (Aβ) generation published during the past two decades, and present a mechanistic explanation for the pathophysiological changes that characterize late-onset AD. We propose that chronic inflammatory conditions cause dysregulation of mechanisms to clear misfolded or damaged neuronal proteins that accumulate with age, and concomitantly lead to tau-associated impairments of axonal integrity and transport. Such changes have several neuropathological consequences: focal accumulation of mitochondria, resulting in metabolic impairments; induction of axonal swelling and leakage, followed by destabilization of synaptic contacts; deposition of amyloid precursor protein in swollen neurites, and generation of aggregation-prone peptides; further tau hyperphosphorylation, ultimately resulting in neurofibrillary tangle formation and neuronal death. The proposed sequence of events provides a link between Aβ and tau-related neuropathology, and underscores the concept that degenerating neurites represent a cause rather than a consequence of Aβ accumulation in late-onset AD.
Key Points
-
Despite tremendous investments in basic and clinical research, no cure or preventive treatment for Alzheimer disease (AD) exists
-
A re-evaluation of the current view of the mechanisms underlying late-onset AD pathology is a prerequisite for future translational approaches
-
Inflammatory processes are strongly correlated with AD onset and progression in humans, and could have a pivotal role in disease aetiology
-
Chronic inflammation coupled with neuronal ageing induces cellular stress and concomitant impairments in basic neuronal functions
-
Inflammation-induced hyperphosphorylation and missorting of tau might represent one of the earliest neuropathological changes in late-onset AD
-
Molecular changes underlying late-onset AD involve impairments in cytoskeleton stability and axonal transport, which could trigger axonal degeneration and formation of senile plaques and neurofibrillary tangles, resulting in neuronal death
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ferri, C. P. et al. Global prevalence of dementia: a Delphi consensus study. Lancet 366, 2112–2117 (2005).
Castellani, R. J., Rolston, R. K. & Smith, M. A. Alzheimer disease. Dis. Mon. 56, 484–546 (2010).
Serrano-Pozo, A., Frosch, M. P., Masliah, E. & Hyman, B. T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 1, a006189 (2011).
Hardy, J. A. & Higgins, G. A. Alzheimer's disease: the amyloid cascade hypothesis. Science 256, 184–185 (1992).
Herrup, K. Reimagining Alzheimer's disease—an age-based hypothesis. J. Neurosci. 30, 16755–16762 (2010).
Corder, E. H. et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261, 921–923 (1993).
Harold, D. et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat. Genet. 41, 1088–1093 (2009).
Lambert, J. C. et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat. Genet. 41, 1094–1099 (2009).
Gerrish, A. et al. The role of variation at AβPP, PSEN1, PSEN2, and MAPT in late onset Alzheimer's disease. J. Alzheimers Dis. 28, 377–387 (2012).
Cribbs, D. H. et al. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J. Neuroinflammation 9, 179 (2012).
McGeer, P. L. & McGeer, E. G. Local neuroinflammation and the progression of Alzheimer's disease. J. Neurovirol. 8, 529–538 (2002).
Swardfager, W. et al. A meta-analysis of cytokines in Alzheimer's disease. Biol. Psychiatry 68, 930–941 (2010).
Wyss-Coray, T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat. Med. 12, 1005–1015 (2006).
Meyer, U. et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J. Neurosci. 26, 4752–4762 (2006).
Meyer, U. et al. Relative prenatal and postnatal maternal contributions to schizophrenia-related neurochemical dysfunction after in utero immune challenge. Neuropsychopharmacology 33, 441–456 (2008).
Knuesel, I. et al. Age-related accumulation of Reelin in amyloid-like deposits. Neurobiol. Aging 30, 697–716 (2009).
Krstic, D. et al. Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. J. Neuroinflammation 9, 151 (2012).
Holmes, C. et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology 73, 768–774 (2009).
Sheng, J. G. et al. In vivo and in vitro evidence supporting a role for the inflammatory cytokine interleukin-1 as a driving force in Alzheimer pathogenesis. Neurobiol. Aging 17, 761–766 (1996).
Doehner, J., Genoud, C., Imhof, C., Krstic, D. & Knuesel, I. Extrusion of misfolded and aggregated proteins—a protective strategy of aging neurons? Eur. J. Neurosci. 35, 1938–1950 (2012).
Doehner, J., Madhusudan, A., Konietzko, U., Fritschy, J. M. & Knuesel, I. Co-localization of Reelin and proteolytic AβPP fragments in hippocampal plaques in aged wild-type mice. J. Alzheimers Dis. 19, 1339–1357 (2010).
Fiala, J. C., Feinberg, M., Peters, A. & Barbas, H. Mitochondrial degeneration in dystrophic neurites of senile plaques may lead to extracellular deposition of fine filaments. Brain Struct. Funct. 212, 195–207 (2007).
Price, D. L. et al. Aged non-human primates: an animal model of age-associated neurodegenerative disease. Brain Pathol. 1, 287–296 (1991).
Kanaan, N. M. et al. Pathogenic forms of tau inhibit kinesin-dependent axonal transport through a mechanism involving activation of axonal phosphotransferases. J. Neurosci. 31, 9858–9868 (2011).
Shahpasand, K. et al. Regulation of mitochondrial transport and inter-microtubule spacing by tau phosphorylation at the sites hyperphosphorylated in Alzheimer's disease. J. Neurosci. 32, 2430–2441 (2012).
Shemesh, O. A., Erez, H., Ginzburg, I. & Spira, M. E. Tau-induced traffic jams reflect organelles accumulation at points of microtubule polar mismatching. Traffic 9, 458–471 (2008).
Iijima-Ando, K. et al. Loss of axonal mitochondria promotes tau-mediated neurodegeneration and Alzheimer's disease-related tau phosphorylation via PAR-1. PLoS Genet. 8, e1002918 (2012).
Xiao, A. W. et al. The origin and development of plaques and phosphorylated tau are associated with axonopathy in Alzheimer's disease. Neurosci. Bull. 27, 287–299 (2011).
Hoover, B. R. et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 68, 1067–1081 (2010).
Nixon, R. A. et al. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J. Neuropathol. Exp. Neurol. 64, 113–122 (2005).
Nixon, R. A. & Yang, D. S. Autophagy failure in Alzheimer's disease—locating the primary defect. Neurobiol. Dis. 43, 38–45 (2011).
Sergeant, N. et al. Truncated beta-amyloid peptide species in pre-clinical Alzheimer's disease as new targets for the vaccination approach. J. Neurochem. 85, 1581–1591 (2003).
McGeer, P. L. et al. Immunohistochemical localization of beta-amyloid precursor protein sequences in Alzheimer and normal brain tissue by light and electron microscopy. J. Neurosci. Res. 31, 428–442 (1992).
Perry, G. et al. Immunolocalization of the amyloid precursor protein within the senile plaque. Prog. Clin. Biol. Res. 317, 1021–1025 (1989).
Malamud, N. & Hirano, A. Atlas of Neuropathology 2nd edn 314–327 (University of California Press, Berkley, Los Angeles, London, 1974).
Kocherhans, S. et al. Reduced Reelin expression accelerates amyloid-beta plaque formation and tau pathology in transgenic Alzheimer's disease mice. J. Neurosci. 30, 9228–9240 (2010).
McGeer, P. L., Schulzer, M. & McGeer, E. G. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology 47, 425–432 (1996).
Schmidt, R. et al. Early inflammation and dementia: a 25-year follow-up of the Honolulu–Asia Aging Study. Ann. Neurol. 52, 168–174 (2002).
Engelhart, M. J. et al. Inflammatory proteins in plasma and the risk of dementia: the Rotterdam Study. Arch. Neurol. 61, 668–672 (2004).
Dunn, N., Mullee, M., Perry, V. H. & Holmes, C. Association between dementia and infectious disease: evidence from a case-control study. Alzheimer Dis. Assoc. Disord. 19, 91–94 (2005).
Aisen, P. S. et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA 289, 2819–2826 (2003).
Thal, L. J. et al. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology 30, 1204–1215 (2005).
Breitner, J. C. et al. Extended results of the Alzheimer's disease anti-inflammatory prevention trial. Alzheimers Dement. 7, 402–411 (2011).
Crystal, H. et al. Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer's disease. Neurology 38, 1682–1687 (1988).
Snowdon, D. A. Aging and Alzheimer's disease: lessons from the Nun Study. Gerontologist 37, 150–156 (1997).
Lue, L. F., Brachova, L., Civin, W. H. & Rogers, J. Inflammation, Aβ deposition, and neurofibrillary tangle formation as correlates of Alzheimer's disease neurodegeneration. J. Neuropathol. Exp. Neurol. 55, 1083–1088 (1996).
Morimoto, K. et al. Expression profiles of cytokines in the brains of Alzheimer's disease (AD) patients compared to the brains of non-demented patients with and without increasing AD pathology. J. Alzheimers Dis. 25, 59–76 (2011).
Parachikova, A. et al. Inflammatory changes parallel the early stages of Alzheimer disease. Neurobiol. Aging 28, 1821–1833 (2007).
Schwab, C., Hosokawa, M. & McGeer, P. L. Transgenic mice overexpressing amyloid beta protein are an incomplete model of Alzheimer disease. Exp. Neurol. 188, 52–64 (2004).
Maarouf, C. L. et al. Alzheimer's disease and non-demented high pathology control nonagenarians: comparing and contrasting the biochemistry of cognitively successful aging. PLoS One 6, e27291 (2011).
Castellani, R. J. et al. Reexamining Alzheimer's disease: evidence for a protective role for amyloid-β protein precursor and amyloid-β. J. Alzheimers Dis. 18, 447–452 (2009).
Edison, P. et al. Microglia, amyloid, and cognition in Alzheimer's disease: an [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol. Dis. 32, 412–419 (2008).
Yokokura, M. et al. In vivo changes in microglial activation and amyloid deposits in brain regions with hypometabolism in Alzheimer's disease. Eur. J. Nucl. Med. Mol. Imaging 38, 343–351 (2011).
Andersen, K., Lolk, A., Kragh-Sorensen, P., Petersen, N. E. & Green, A. Depression and the risk of Alzheimer disease. Epidemiology 16, 233–238 (2005).
Balakrishnan, K. et al. Plasma Aβ42 correlates positively with increased body fat in healthy individuals. J. Alzheimers Dis. 8, 269–282 (2005).
Biessels, G. J. & Kappelle, L. J. Increased risk of Alzheimer's disease in Type II diabetes: insulin resistance of the brain or insulin-induced amyloid pathology? Biochem. Soc. Trans. 33, 1041–1044 (2005).
Casserly, I. & Topol, E. Convergence of atherosclerosis and Alzheimer's disease: inflammation, cholesterol, and misfolded proteins. Lancet 363, 1139–1146 (2004).
Dowlati, Y. et al. A meta-analysis of cytokines in major depression. Biol. Psychiatry 67, 446–457 (2010).
Kamer, A. R. et al. TNF-α and antibodies to periodontal bacteria discriminate between Alzheimer's disease patients and normal subjects. J. Neuroimmunol. 216, 92–97 (2009).
Ownby, R. L., Crocco, E., Acevedo, A., John, V. & Loewenstein, D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch. Gen. Psychiatry 63, 530–538 (2006).
Tizard, I. Sickness behavior, its mechanisms and significance. Anim. Health Res. Rev. 9, 87–99 (2008).
Zlokovic, B. V. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat. Rev. Neurosci. 12, 723–738 (2011).
Cunningham, C., Campion, S., Teeling, J., Felton, L. & Perry, V. H. The sickness behaviour and CNS inflammatory mediator profile induced by systemic challenge of mice with synthetic double-stranded RNA (poly I:C). Brain Behav. Immun. 21, 490–502 (2007).
Hannestad, J. et al. Endotoxin-induced systemic inflammation activates microglia: [11C]PBR28 positron emission tomography in nonhuman primates. Neuroimage 63, 232–239 (2012).
Pitossi, F., del Rey, A., Kabiersch, A. & Besedovsky, H. Induction of cytokine transcripts in the central nervous system and pituitary following peripheral administration of endotoxin to mice. J. Neurosci. Res. 48, 287–298 (1997).
Anisman, H., Gibb, J. & Hayley, S. Influence of continuous infusion of interleukin-1β on depression-related processes in mice: corticosterone, circulating cytokines, brain monoamines, and cytokine mRNA expression. Psychopharmacology (Berl.) 199, 231–244 (2008).
Lemstra, A. W. et al. Microglia activation in sepsis: a case–control study. J. Neuroinflammation 4, 4 (2007).
Puentener, U., Booth, S. G., Perry, V. H. & Teeling, J. L. Long-term impact of systemic bacterial infection on the cerebral vasculature and microglia. J. Neuroinflammation 9, 146 (2012).
Lee, C. K., Weindruch, R. & Prolla, T. A. Gene-expression profile of the ageing brain in mice. Nat. Genet. 25, 294–297 (2000).
Lu, T. et al. Gene regulation and DNA damage in the ageing human brain. Nature 429, 883–891 (2004).
Franceschi, C. et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 128, 92–105 (2007).
Cunningham, C. & Maclullich, A. M. At the extreme end of the psychoneuroimmunological spectrum: delirium as a maladaptive sickness behaviour response. Brain. Behav. Immun. http://dx.doi.org/10.1016/j.bbi.2012.07.012.
Norden, D. M. & Godbout, J. P. Microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol. Appl. Neurobiol. http://dx.doi.org/10.1111/j.1365-2990.2012.01306.x.
Wynne, A. M., Henry, C. J. & Godbout, J. P. Immune and behavioral consequences of microglial reactivity in the aged brain. Integr. Comp. Biol. 49, 254–266 (2009).
Barrientos, R. M. et al. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol. Aging 27, 723–732 (2006).
Godbout, J. P. et al. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 19, 1329–1331 (2005).
Barrientos, R. M. et al. Time course of hippocampal IL-1 β and memory consolidation impairments in aging rats following peripheral infection. Brain. Behav. Immun. 23, 46–54 (2009).
Henry, C. J., Huang, Y., Wynne, A. M. & Godbout, J. P. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1β and anti-inflammatory IL-10 cytokines. Brain. Behav. Immun. 23, 309–317 (2009).
Lee, C. Y. & Landreth, G. E. The role of microglia in amyloid clearance from the AD brain. J. Neural Transm. 117, 949–960 (2010).
Grathwohl, S. A. et al. Formation and maintenance of Alzheimer's disease β-amyloid plaques in the absence of microglia. Nat. Neurosci. 12, 1361–1363 (2009).
Chung, H., Brazil, M. I., Soe, T. T. & Maxfield, F. R. Uptake, degradation, and release of fibrillar and soluble forms of Alzheimer's amyloid β-peptide by microglial cells. J. Biol. Chem. 274, 32301–32308 (1999).
Njie, E. G. et al. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol. Aging 33, 195.e1–195.e12 (2012).
Sheng, J. G., Mrak, R. E. & Griffin, W. S. Neuritic plaque evolution in Alzheimer's disease is accompanied by transition of activated microglia from primed to enlarged to phagocytic forms. Acta Neuropathol. 94, 1–5 (1997).
Peri, F. & Nusslein-Volhard, C. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell 133, 916–927 (2008).
McGeer, P. L., Itagaki, S., Tago, H. & McGeer, E. G. Occurrence of HLA-DR reactive microglia in Alzheimer's disease. Ann. NY Acad. Sci. 540, 319–323 (1988).
Streit, W. J., Braak, H., Xue, Q. S. & Bechmann, I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer's disease. Acta Neuropathol. 118, 475–485 (2009).
Hoozemans, J. J., Rozemuller, A. J., van Haastert, E. S., Eikelenboom, P. & van Gool, W. A. Neuroinflammation in Alzheimer's disease wanes with age. J. Neuroinflammation 8, 171 (2011).
Bhaskar, K. et al. Regulation of tau pathology by the microglial fractalkine receptor. Neuron 68, 19–31 (2010).
Gorlovoy, P., Larionov, S., Pham, T. T. & Neumann, H. Accumulation of tau induced in neurites by microglial proinflammatory mediators. FASEB J. 23, 2502–2513 (2009).
Li, Y., Liu, L., Barger, S. W. & Griffin, W. S. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J. Neurosci. 23, 1605–1611 (2003).
Sarlus, H. et al. Allergy influences the inflammatory status of the brain and enhances tau phosphorylation. J. Cell. Mol. Med. 16, 2401–2412 (2012).
Johnson, G. V. & Stoothoff, W. H. Tau phosphorylation in neuronal cell function and dysfunction. J. Cell Sci. 117, 5721–5729 (2004).
Fulga, T. A. et al. Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nat. Cell Biol. 9, 139–148 (2007).
Iqbal, K. et al. Defective brain microtubule assembly in Alzheimer's disease. Lancet 2, 421–426 (1986).
Terry, R. D. The pathogenesis of Alzheimer disease: an alternative to the amyloid hypothesis. J. Neuropathol. Exp. Neurol. 55, 1023–1025 (1996).
Praprotnik, D., Smith, M. A., Richey, P. L., Vinters, H. V. & Perry, G. Filament heterogeneity within the dystrophic neurites of senile plaques suggests blockage of fast axonal transport in Alzheimer's disease. Acta Neuropathol. 91, 226–235 (1996).
Stokin, G. B. & Goldstein, L. S. Axonal transport and Alzheimer's disease. Ann. Rev. Biochem. 75, 607–627 (2006).
Stieber, A., Mourelatos, Z. & Gonatas, N. K. In Alzheimer's disease the Golgi apparatus of a population of neurons without neurofibrillary tangles is fragmented and atrophic. Am. J. Pathol. 148, 415–426 (1996).
Lazarov, O. et al. Impairments in fast axonal transport and motor neuron deficits in transgenic mice expressing familial Alzheimer's disease-linked mutant presenilin 1. J. Neurosci. 27, 7011–7020 (2007).
Pigino, G., Pelsman, A., Mori, H. & Busciglio, J. Presenilin-1 mutations reduce cytoskeletal association, deregulate neurite growth, and potentiate neuronal dystrophy and tau phosphorylation. J. Neurosci. 21, 834–842 (2001).
Rodrigues, E. M., Weissmiller, A. M. & Goldstein, L. S. Enhanced β-secretase processing alters APP axonal transport and leads to axonal defects. Hum. Mol. Genet. http://dx.doi.org/10.1093/hmg/dds297.
Tesseur, I. et al. Prominent axonopathy and disruption of axonal transport in transgenic mice expressing human apolipoprotein E4 in neurons of brain and spinal cord. Am. J. Pathol. 157, 1495–1510 (2000).
Braak, H., Thal, D. R., Ghebremedhin, E. & Del Tredici, K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 70, 960–969 (2011).
Ma, X. M. & Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 10, 307–318 (2009).
Seifert, U. et al. Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell 142, 613–624 (2010).
Gavilan, M. P. et al. Molecular and cellular characterization of the age-related neuroinflammatory processes occurring in normal rat hippocampus: potential relation with the loss of somatostatin GABAergic neurons. J. Neurochem. 103, 984–996 (2007).
Pintado, C. et al. Lipopolysaccharide-induced neuroinflammation leads to the accumulation of ubiquitinated proteins and increases susceptibility to neurodegeneration induced by proteasome inhibition in rat hippocampus. J. Neuroinflammation 9, 87 (2012).
Forloni, G., Demicheli, F., Giorgi, S., Bendotti, C. & Angeretti, N. Expression of amyloid precursor protein mRNAs in endothelial, neuronal and glial cells: modulation by interleukin-1. Brain Res. Mol. Brain Res. 16, 128–134 (1992).
Sheng, J. G. et al. Lipopolysaccharide-induced-neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid β peptide in APPswe transgenic mice. Neurobiol. Dis. 14, 133–145 (2003).
Griffin, W. S. et al. Microglial interleukin-1 alpha expression in human head injury: correlations with neuronal and neuritic beta-amyloid precursor protein expression. Neurosci. Lett. 176, 133–136 (1994).
Itoh, T. et al. Expression of amyloid precursor protein after rat traumatic brain injury. Neurol. Res. 31, 103–109 (2009).
Johnson, V. E., Stewart, W. & Smith, D. H. Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathol. 22, 142–149 (2012).
Mouzon, B. C. et al. Repetitive mild traumatic brain injury in a mouse model produces learning and memory deficits accompanied by histological changes. J. Neurotrauma http://dx.doi.org/10.1089/neu.2012.2498.
Groemer, T. W. et al. Amyloid precursor protein is trafficked and secreted via synaptic vesicles. PLoS One 6, e18754 (2011).
Koo, E. H. et al. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc. Natl Acad. Sci. USA 87, 1561–1565 (1990).
Morales-Corraliza, J. et al. In vivo turnover of tau and APP metabolites in the brains of wild-type and Tg2576 mice: greater stability of sAPP in the β-amyloid depositing mice. PLoS One 4, e7134 (2009).
Ichihara, N. et al. Axonal degeneration promotes abnormal accumulation of amyloid β-protein in ascending gracile tract of gracile axonal dystrophy (GAD) mouse. Brain Res. 695, 173–178 (1995).
Lee, S., Sato, Y. & Nixon, R. A. Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer's-like axonal dystrophy. J. Neurosci. 31, 7817–7830 (2011).
Weyer, S. W. et al. APP and APLP2 are essential at PNS and CNS synapses for transmission, spatial learning and LTP. EMBO J. 30, 2266–2280 (2011).
Stokin, G. B. et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer's disease. Science 307, 1282–1288 (2005).
Wirths, O., Weis, J., Szczygielski, J., Multhaup, G. & Bayer, T. A. Axonopathy in an APP/PS1 transgenic mouse model of Alzheimer's disease. Acta Neuropathol. 111, 312–319 (2006).
Martin, L. J., Pardo, C. A., Cork, L. C. & Price, D. L. Synaptic pathology and glial responses to neuronal injury precede the formation of senile plaques and amyloid deposits in the aging cerebral cortex. Am. J. Pathol. 145, 1358–1381 (1994).
Yu, W. H. et al. Macroautophagy—a novel β-amyloid peptide-generating pathway activated in Alzheimer's disease. J. Cell. Biol. 171, 87–98 (2005).
Cataldo, A. M. & Nixon, R. A. Enzymatically active lysosomal proteases are associated with amyloid deposits in Alzheimer brain. Proc. Natl Acad. Sci. USA 87, 3861–3865 (1990).
Schechter, I. & Ziv, E. Cathepsins S, B and L with aminopeptidases display β-secretase activity associated with the pathogenesis of Alzheimer's disease. Biol. Chem. 392, 555–569 (2011).
Jin, M. et al. Soluble amyloid β-protein dimers isolated from Alzheimer cortex directly induce tau hyperphosphorylation and neuritic degeneration. Proc. Natl Acad. Sci. USA 108, 5819–5824 (2011).
Brecht, W. J. et al. Neuron-specific apolipoprotein E4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J. Neurosci. 24, 2527–2534 (2004).
Zhou, W., Scott, S. A., Shelton, S. B. & Crutcher, K. A. Cathepsin D-mediated proteolysis of apolipoprotein E: possible role in Alzheimer's disease. Neuroscience 143, 689–701 (2006).
Adalbert, R. et al. Severely dystrophic axons at amyloid plaques remain continuous and connected to viable cell bodies. Brain 132, 402–416 (2009).
Scheff, S. W., Price, D. A., Schmitt, F. A. & Mufson, E. J. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiol. Aging 27, 1372–1384 (2006).
Misko, A. L., Sasaki, Y., Tuck, E., Milbrandt, J. & Baloh, R. H. Mitofusin2 mutations disrupt axonal mitochondrial positioning and promote axon degeneration. J. Neurosci. 32, 4145–4155 (2012).
Ittner, L. M. & Gotz, J. Amyloid-β and tau—a toxic pas de deux in Alzheimer's disease. Nat. Rev. Neurosci. 12, 65–72 (2011).
Yilmazer-Hanke, D. M. & Hanke, J. Progression of Alzheimer-related neuritic plaque pathology in the entorhinal region, perirhinal cortex and hippocampal formation. Dement. Geriatr. Cogn. Disord. 10, 70–76 (1999).
Schmidt, M. L., DiDario, A. G., Lee, V. M. & Trojanowski, J. Q. An extensive network of PHF tau-rich dystrophic neurites permeates neocortex and nearly all neuritic and diffuse amyloid plaques in Alzheimer disease. FEBS Lett. 344, 69–73 (1994).
de Calignon, A. et al. Caspase activation precedes and leads to tangles. Nature 464, 1201–1204 (2010).
Buki, A., Okonkwo, D. O., Wang, K. K. & Povlishock, J. T. Cytochrome c release and caspase activation in traumatic axonal injury. J. Neurosci. 20, 2825–2834 (2000).
Rohn, T. T. et al. Caspase-9 activation and caspase cleavage of tau in the Alzheimer's disease brain. Neurobiol. Dis. 11, 341–354 (2002).
Leroy, K. et al. Early axonopathy preceding neurofibrillary tangles in mutant tau transgenic mice. Am. J. Pathol. 171, 976–992 (2007).
Acknowledgements
This study was supported by the Swiss National Science Foundation, grant number 310030-132629, the Gottfried und Julia Bangerter-Rhyner Foundation, and the Olga Mayenfisch Foundation.
Author information
Authors and Affiliations
Contributions
Both authors contributed to researching data for the article, discussions of the content, writing the article and to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Krstic, D., Knuesel, I. Deciphering the mechanism underlying late-onset Alzheimer disease. Nat Rev Neurol 9, 25–34 (2013). https://doi.org/10.1038/nrneurol.2012.236
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2012.236
This article is cited by
-
Assessment of the protective and ameliorative impact of quercetin nanoparticles against neuronal damage induced in the hippocampus by acrolein
Beni-Suef University Journal of Basic and Applied Sciences (2024)
-
Mind the Gap: Unraveling the Intricate Dance Between Alzheimer’s Disease and Related Dementias and Bone Health
Current Osteoporosis Reports (2024)
-
Nisin a probiotic bacteriocin mitigates brain microbiome dysbiosis and Alzheimer’s disease-like neuroinflammation triggered by periodontal disease
Journal of Neuroinflammation (2023)
-
Therapeutic approaches using natural substances on the streptozotocin-induced animal model of sporadic Alzheimer’s disease: a systematic review
Advances in Traditional Medicine (2023)
-
Cognitive Deficits and Alzheimer’s Disease-Like Pathologies in the Aged Chinese Tree Shrew
Molecular Neurobiology (2023)