Abstract
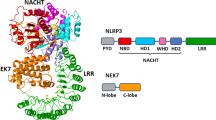
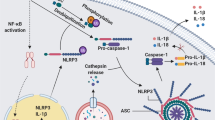
Inflammasomes are key inducers of inflammation in response to exogenous and endogenous stimuli, because they regulate the processing and secretion of the proinflammatory cytokines IL-1β and IL-18. Thus, inflammasomes have a crucial role in host defence against infection, but they can also be involved in inflammatory diseases. Indeed, the NLRP3 (NOD-, LRR- and pyrin domain-containing 3) inflammasome has been shown to play a part in several inflammatory rheumatic disorders, although the mechanisms involved are better elucidated in some of these diseases than in others. In particular, the pathogenesis of cryopyrin-associated periodic syndromes and microcrystal-induced arthritides is thought to be dependent on activation of the NLRP3 inflammasome, and IL-1 inhibition has shown efficacy as a therapeutic strategy in both groups of conditions. In this Review, we describe the current understanding of the mechanisms that trigger the inflammasome, and consider the relevance of the inflammasome to a variety of rheumatic diseases. In addition, we discuss the current therapies targeting this molecular complex, as well as future therapeutic prospects.
Key Points
-
Inflammasomes are the principal mediators of IL-1β and IL-18 release by monocytes and macrophages
-
Inflammasomes are activated by a large number of cellular factors, such as calcium, potassium efflux, reactive oxygen species and ATP; this list continues to expand
-
A link between inflammasome activation and disease has been clearly established for cryopyrin-associated periodic syndromes and microcrystal-induced arthritides such as gout and basic calcium phosphate-induced calcific tendinitis
-
Several inflammatory rheumatic diseases respond to IL-1 inhibitors, which suggests that dysregulation of IL-1β production might be pathogenic
-
IL-1 inhibitors have been the main treatment for inflammasome-mediated diseases, but there might be other pathways that can be targeted therapeutically
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-1β. Mol. Cell 10, 417–426 (2002).
Franchi, L., Munoz-Planillo, R. & Nunez, G. Sensing and reacting to microbes through the inflammasomes. Nature Immunol. 13, 325–332 (2012).
Gross, O., Thomas, C. J., Guarda, G. & Tschopp, J. The inflammasome: an integrated view. Immunol. Rev. 243, 136–151 (2011).
Strowig, T., Henao-Mejia, J., Elinav, E. & Flavell, R. Inflammasomes in health and disease. Nature 481, 278–286 (2012).
Murakami, T. et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl Acad. Sci. USA 109, 11282–11287 (2012).
Shimada, K. et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36, 401–414 (2012).
Bauernfeind, F. G. et al. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 (2009).
Willingham, S. B. et al. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J. Immunol. 183, 2008–2015 (2009).
Miller, A. F. Superoxide dismutases: ancient enzymes and new insights. FEBS Lett. 586, 585–595 (2012).
Menu, P. et al. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death Dis. 3, e261 (2012).
Naik, E. & Dixit, V. M. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J. Exp. Med. 208, 417–420 (2011).
Rubartelli, A., Gattorno, M., Netea, M. G. & Dinarello, C. A. Interplay between redox status and inflammasome activation. Trends Immunol. 32, 559–566 (2011).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
Jin, C. et al. NLRP3 inflammasome plays a critical role in the pathogenesis of hydroxyapatite-associated arthropathy. Proc. Natl Acad. Sci. USA 108, 14867–14872 (2011).
Pazar, B. et al. Basic calcium phosphate crystals induce monocyte/macrophage IL-1β secretion through the NLRP3 inflammasome in vitro. J. Immunol. 186, 2495–2502 (2011).
Cassel, S. L. et al. The Nalp3 inflammasome is essential for the development of silicosis. Proc. Natl Acad. Sci. USA 105, 9035–9040 (2008).
Dostert, C. et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–677 (2008).
Bylund, J. et al. Enhanced inflammatory responses of chronic granulomatous disease leukocytes involve ROS-independent activation of NF-κB. Eur. J. Immunol. 37, 1087–1096 (2007).
van de Veerdonk, F. L. et al. Reactive oxygen species-independent activation of the IL-1β inflammasome in cells from patients with chronic granulomatous disease. Proc. Natl Acad. Sci. USA 107, 3030–3033 (2010).
Meissner, F., Molawi, K. & Zychlinsky, A. Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat. Immunol. 9, 866–872 (2008).
Zhou, R., Tardivel, A., Thorens, B., Choi, I. & Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 11, 136–140 (2010).
Pelegrin, P., Barroso-Gutierrez, C. & Surprenant, A. P2X7 receptor differentially couples to distinct release pathways for IL-1β in mouse macrophage. J. Immunol. 180, 7147–7157 (2008).
Kanneganti, T. D. et al. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 26, 433–443 (2007).
Petrilli, V. et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 14, 1583–1589 (2007).
Di Virgilio, F. Liaisons dangereuses: P2X7 and the inflammasome. Trends Pharmacol. Sci. 28, 465–472 (2007).
Lee, G. S. et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 492, 123–127 (2012).
Rossol, M. et al. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat. Commun. 3, 1329 (2012).
Shenoy, A. R. et al. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science 336, 481–485 (2012).
Lu, B. et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature 488, 670–674 (2012).
Lachmann, H. J. et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N. Engl. J. Med. 360, 2416–2425 (2009).
Hawkins, P. N., Lachmann, H. J., Aganna, E. & McDermott, M. F. Spectrum of clinical features in Muckle-Wells syndrome and response to anakinra. Arthritis Rheum. 50, 607–612 (2004).
Aksentijevich, I. et al. The clinical continuum of cryopyrinopathies: novel CIAS1 mutations in North American patients and a new cryopyrin model. Arthritis Rheum. 56, 1273–1285 (2007).
Tassi, S. et al. Altered redox state of monocytes from cryopyrin-associated periodic syndromes causes accelerated IL-1β secretion. Proc. Natl Acad. Sci. USA 107, 9789–9794 (2010).
Hawkins, P. N., Lachmann, H. J. & McDermott, M. F. Interleukin-1-receptor antagonist in the Muckle-Wells syndrome. N. Engl. J. Med. 348, 2583–2584 (2003).
Alexander, T. et al. Successful treatment of acute visual loss in Muckle-Wells syndrome with interleukin 1 receptor antagonist. Ann. Rheum. Dis. 64, 1245–1246 (2005).
Hoffman, H. M. et al. Efficacy and safety of rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studies. Arthritis Rheum. 58, 2443–2452 (2008).
Hoffman, H. M. et al. Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet 364, 1779–1785 (2004).
Agostini, L. et al. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20, 319–325 (2004).
Brydges, S. D. et al. Inflammasome-mediated disease animal models reveal roles for innate but not adaptive immunity. Immunity 30, 875–887 (2009).
Meng, G., Zhang, F., Fuss, I., Kitani, A. & Strober, W. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity 30, 860–874 (2009).
Bonar, S. L. et al. Constitutively activated NLRP3 inflammasome causes inflammation and abnormal skeletal development in mice. PLoS ONE 7, e35979 (2012).
Tanaka, N. et al. High incidence of NLRP3 somatic mosaicism in patients with chronic infantile neurologic, cutaneous, articular syndrome: results of an International Multicenter Collaborative Study. Arthritis Rheum. 63, 3625–3632 (2011).
Ter Haar, N. et al. Treatment of autoinflammatory diseases: results from the Eurofever Registry and a literature review. Ann. Rheum. Dis. doi:10.1136/annrheumdis-2011-201268.
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
So, A., De Smedt, T., Revaz, S. & Tschopp, J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res. Ther. 9, R28 (2007).
So, A., et al. Canakinumab for the treatment of acute flares in difficult-to-treat gouty arthritis: Results of a multicenter, phase II, dose-ranging study. Arthritis Rheum. 62, 3064–3076 (2010).
Terkeltaub, R. et al. The interleukin 1 inhibitor rilonacept in treatment of chronic gouty arthritis: results of a placebo-controlled, monosequence crossover, non-randomised, single-blind pilot study. Ann. Rheum. Dis. 68, 1613–1617 (2009).
Schumacher, H. R. Jr et al. Rilonacept (interleukin-1 trap) for prevention of gout flares during initiation of uric acid-lowering therapy: results from a phase III randomized, double-blind, placebo-controlled, confirmatory efficacy study. Arthritis Care Res. 64, 1462–1470 (2012).
Schlesinger, N. et al. Canakinumab reduces the risk of acute gouty arthritis flares during initiation of allopurinol treatment: results of a double-blind, randomised study. Ann. Rheum. Dis. 70, 1264–1271 (2011).
Announ, N., Palmer, G., Guerne, P. A. & Gabay, C. Anakinra is a possible alternative in the treatment and prevention of acute attacks of pseudogout in end-stage renal failure. Joint Bone Spine 76, 424–426 (2009).
Zufferey, P. & So, A. A pilot study of IL-1 inhibition in acute calcific periarthritis of the shoulder. Ann. Rheum. Dis. 72, 465–467 (2013).
Pascual, E., Batlle-Gualda, E., Martinez, A., Rosas, J. & Vela, P. Synovial fluid analysis for diagnosis of intercritical gout. Ann. Intern. Med. 131, 756–759 (1999).
Joosten, L. A. et al. Engagement of fatty acids with Toll-like receptor 2 drives interleukin-1β production via the ASC/caspase 1 pathway in monosodium urate monohydrate crystal-induced gouty arthritis. Arthritis Rheum. 62, 3237–3248 (2010).
Zhang, Y. et al. Purine-rich foods intake and recurrent gout attacks. Ann. Rheum. Dis. 71, 1448–1453 (2012).
Choi, H. K., Atkinson, K., Karlson, E. W., Willett, W. & Curhan, G. Alcohol intake and risk of incident gout in men: a prospective study. Lancet 363, 1277–1281 (2004).
Dinarello, C. A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117, 3720–3732 (2011).
Fontalba, A. et al. Deficiency of the NF-κB inhibitor caspase activating and recruitment domain 8 in patients with rheumatoid arthritis is associated with disease severity. J. Immunol. 179, 4867–4873 (2007).
Kastbom, A. et al. Genetic variation in proteins of the cryopyrin inflammasome influences susceptibility and severity of rheumatoid arthritis (the Swedish TIRA project). Rheumatology 47, 415–417 (2008).
Kastbom, A., Johansson, M., Verma, D., Soderkvist, P. & Rantapaa-Dahlqvist, S. CARD8 p.C10X polymorphism is associated with inflammatory activity in early rheumatoid arthritis. Ann. Rheum. Dis. 69, 723–726 (2010).
Ben Hamad, M. et al. Association study of CARD8 (p.C10X) and NLRP3 (p.Q705K) variants with rheumatoid arthritis in French and Tunisian populations. Int. J. Immunogenet. 39, 131–136 (2012).
Blomgran, R. et al. Common genetic variations in the NALP3 inflammasome are associated with delayed apoptosis of human neutrophils. PLoS ONE 7, e31326 (2012).
Verma, D. et al. The Q705K polymorphism in NLRP3 is a gain-of-function alteration leading to excessive interleukin-1β and IL-18 production. PLoS ONE 7, e34977 (2012).
Kolly, L. et al. Inflammatory role of ASC in antigen-induced arthritis is independent of caspase-1, NALP-3, and IPAF. J. Immunol. 183, 4003–4012 (2009).
Ippagunta, S. K. et al. Inflammasome-independent role of apoptosis-associated speck-like protein containing a CARD (ASC) in T cell priming is critical for collagen-induced arthritis. J. Biol. Chem. 285, 12454–12462 (2010).
Yamazaki, H. et al. ASC plays a role in the priming phase of the immune response to type II collagen in collagen-induced arthritis. Rheumatol. Int. 32, 1625–1632 (2012).
Hasegawa, M. et al. ASC-mediated NF-κB activation leading to interleukin-8 production requires caspase-8 and is inhibited by CLARP. J. Biol. Chem. 280, 15122–15130 (2005).
Hasegawa, M. et al. Mechanism and repertoire of ASC-mediated gene expression. J. Immunol. 182, 7655–7662 (2009).
Joosten, L. A. et al. Inflammatory arthritis in caspase 1 gene-deficient mice: Contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1β. Arthritis Rheum. 60, 3651–3662 (2009).
Guma, M. et al. Caspase 1-independent activation of interleukin-1β in neutrophil-predominant inflammation. Arthritis Rheum. 60, 3642–3650 (2009).
Kolly, L. et al. Expression and function of the NALP3 inflammasome in rheumatoid synovium. Immunology 129, 178–185 (2010).
Rosengren, S., Hoffman, H. M., Bugbee, W. & Boyle, D. L. Expression and regulation of cryopyrin and related proteins in rheumatoid arthritis synovium. Ann. Rheum. Dis. 64, 708–714 (2005).
van den Berg, W. B. Arguments for interleukin 1 as a target in chronic arthritis. Ann. Rheum. Dis. 59 (Suppl. 1), i81–i84 (2000).
Fuerst, M. et al. Calcification of articular cartilage in human osteoarthritis. Arthritis Rheum. 60, 2694–2703 (2009).
Bougault, C. et al. Stress-induced cartilage degradation does not depend on NLRP3 inflammasome in osteoarthritis. Arthritis Rheum. 64, 3972–3981 (2012).
Said-Sadier, N., Padilla, E., Langsley, G. & Ojcius, D. M. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS ONE 5, e10008 (2010).
Hise, A. G. et al. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 5, 487–497 (2009).
Lamkanfi, M., Malireddi, R. K. & Kanneganti, T. D. Fungal zymosan and mannan activate the cryopyrin inflammasome. J. Biol. Chem. 284, 20574–20581 (2009).
Oosting, M. et al. Borrelia species induce inflammasome activation and IL-17 production through a caspase-1-dependent mechanism. Eur. J. Immunol. 41, 172–181 (2011).
Pascual, V., Allantaz, F., Arce, E., Punaro, M. & Banchereau, J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J. Exp. Med. 201, 1479–1486 (2005).
Naumann, L. et al. IL1-receptor antagonist anakinra provides long-lasting efficacy in the treatment of refractory adult-onset Still's disease. Ann. Rheum. Dis. 69, 466–467 (2010).
Krause, K. et al. Efficacy and safety of the interleukin-1 antagonist rilonacept in Schnitzler syndrome: an open-label study. Allergy 67, 943–950 (2012).
Pascual, V. et al. How the study of children with rheumatic diseases identified interferon-α and interleukin-1 as novel therapeutic targets. Immunol. Rev. 223, 39–59 (2008).
Kone-Paut, I., Sanchez, E., Le Quellec, A., Manna, R. & Touitou, I. Autoinflammatory gene mutations in Behcet's disease. Ann. Rheum. Dis. 66, 832–834 (2007).
Pizzirani, C. et al. Dysfunctional inflammasome in Schnitzler's syndrome. Rheumatology 48, 1304–1308 (2009).
Lachmann, H. J., Quartier, P., So, A. & Hawkins, P. N. The emerging role of interleukin-1β in autoinflammatory diseases. Arthritis Rheum. 63, 314–324 (2011).
Schlesinger, N. et al. Canakinumab for acute gouty arthritis in patients with limited treatment options: results from two randomised, multicentre, active-controlled, double-blind trials and their initial extensions. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2011-200908.
Gross, O. et al. Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity 36, 388–400 (2012).
Narayan, S. et al. Octacalcium phosphate crystals induce inflammation in vivo through interleukin-1 but independent of the NLRP3 inflammasome in mice. Arthritis Rheum. 63, 422–433 (2011).
Wannamaker, W. et al. (S)-1-(S)-2-{[1-(4-amino-3-chloro-phenyl)-methanoyl]-amino}-3,3-dimethyl-butanoyl)-pyrrolidine-2-carboxylic acid (2R,3S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide (VX-765), an orally available selective interleukin (IL)-converting enzyme/caspase-1 inhibitor, exhibits potent anti-inflammatory activities by inhibiting the release of IL-1β and IL-18. J. Pharmacol. Exp. Ther. 321, 509–516 (2007).
Abdollahi-Roodsaz, S. et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J. Clin. Invest. 118, 205–216 (2008).
Kovarova, M. et al. NLRP1-dependent pyroptosis leads to acute lung injury and morbidity in mice. J. Immunol. 189, 2006–2016 (2012).
Levinsohn, J. L. et al. Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome. PLoS Pathog. 8, e1002638 (2012).
Saiga, H. et al. Critical role of AIM2 in Mycobacterium tuberculosis infection. Int. Immunol. 24, 637–644 (2012).
Pierini, R. et al. AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ. 19, 1709–1721 (2012).
Acknowledgements
We acknowledge the support of the Swiss National Science Foundation (Grant 310030_130085), the Fondation Warnery and the Institute of Arthritis Research.
Author information
Authors and Affiliations
Contributions
A. So contributed to researching data for the article, discussions of content, writing the article and reviewing/editing the manuscript. A. Ives and N. Busso contributed to researching data for the article, writing the article and reviewing/editing the manuscript. L. A. B. Joosten contributed to discussions of content and writing the article.
Corresponding author
Ethics declarations
Competing interests
A. So has received honoraria from Novartis AG for participation in advisory boards and consultant fees. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
So, A., Ives, A., Joosten, L. et al. Targeting inflammasomes in rheumatic diseases. Nat Rev Rheumatol 9, 391–399 (2013). https://doi.org/10.1038/nrrheum.2013.61
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2013.61
This article is cited by
-
Hydroxychloroquine inhibits IL-1β production from amyloid-stimulated human neutrophils
Arthritis Research & Therapy (2019)
-
IL-1β Enhances Wnt Signal by Inhibiting DKK1
Inflammation (2018)
-
What rheumatologists need to know about CRISPR/Cas9
Nature Reviews Rheumatology (2017)
-
The inflammasome in fibromyalgia and CRPS: a microglial hypothesis?
Nature Reviews Rheumatology (2015)
-
CASPASE-12 and rheumatoid arthritis in African-Americans
Immunogenetics (2014)