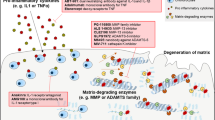
Key Points
-
Inflammation and inflammation-induced catabolism (for example, involving activation of matrix metalloproteinases) are tightly controlled by Toll-like-receptor-mediated innate immune responses
-
Toll-like-receptor 4 (TLR4) binds a number of different agonists, some of which (so-called damage-associated molecular patterns) are released when tissues are damaged
-
The expression of TLR4 in cartilage is increased throughout the development of osteoarthritis (OA)
-
Many TLR4 agonists that have been identified in the joints of patients with OA can induce inflammatory responses in ex vivo tissue samples fromthese patients
-
Several pathways modulate TLR4 signalling in joint tissues, and a number of TLR4 blockers might be candidate disease-modifying OA drugs (DMOADs)
Abstract
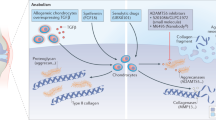
Osteoarthritis (OA), the most common rheumatic disease, is characterized by joint-space narrowing due to progressive cartilage degradation and alterations in subchondral bone and the synovial membrane. These articular disturbances can have severe consequences, including pain, disability and loss of joint architectural integrity. Although the aetiology of OA is not understood, chondrocyte-mediated inflammatory responses triggered by the activation of innate immune receptors by damage-associated molecules are thought to be involved. In this Review, we examine the relationship between Toll-like receptor 4 (TLR4) and OA in cartilage as well as in other OA-affected tissues, such as subchondral bone and synovium. We also discuss the different TLR4 agonists associated with OA and their effects in joint tissues. Finally, we describe existing and novel strategies that might be used to develop TLR4-specific disease-modifying OA drugs (DMOADs).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dvinge, H. & Bertone, P. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage 13, 769–781 (2005).
Reynard, L. N. & Loughlin, J. Genetics and epigenetics of osteoarthritis. Maturitas 71, 200–204 (2012).
Gómez, R. et al. What's new in our understanding of the role of adipokines in rheumatic diseases? Nat. Rev. Rheumatol. 7, 528–536 (2011).
Herrero-Beaumont, G. & Roman-Blas, J. A. Osteoarthritis: Osteoporotic OA: a reasonable target for bone-acting agents. Nat. Rev. Rheumatol. 9, 448–450 (2013).
Scanzello, C. R., Plaas, A. & Crow, M. K. Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr. Opin. Rheumatol. 20, 565–572 (2008).
Janeway, C. A. Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54 (Pt 1), 1–13 (1989).
Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1, 135–145 (2001).
Abdollahi-Roodsaz, S., van de Loo, F. A. & van den Berg, W. B. Trapped in a vicious loop: Toll-like receptors sustain the spontaneous cytokine production by rheumatoid synovium. Arthritis Res. Ther. 13, 105 (2011).
Goldring, S. R. & Scanzello, C. R. Plasma proteins take their toll on the joint in osteoarthritis. Arthritis Res. Ther. 14, 111 (2012).
Chen, K. et al. Toll-like receptors in inflammation, infection and cancer. Int. Immunopharmacol. 7, 1271–1285 (2007).
Keshava Prasad, T. S. et al. Human Protein Reference Database—2009 update. Nucleic Acids Res. 37, D767–D772 (2009).
Medzhitov, R., Preston-Hurlburt, P. & Janeway, C. A. Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388, 394–397 (1997).
Chaturvedi, A. & Pierce, S. K. How location governs Toll-like receptor signaling. Traffic 10, 621–628 (2009).
Gangloff, M. Different dimerisation mode for TLR4 upon endosomal acidification? Trends Biochem. Sci. 37, 92–98 (2012).
Da Silva Correia, J. & Ulevitch, R. J. MD-2 and TLR4 N-linked glycosylations are important for a functional lipopolysaccharide receptor. J. Biol. Chem. 277, 1845–1854 (2002).
Raijmakers, R., Kraiczek, K., de Jong, A. P., Mohammed, S. & Heck, A. J. Exploring the human leukocyte phosphoproteome using a microfluidic reversed-phase-TiO2-reversed-phase high-performance liquid chromatography phosphochip coupled to a quadrupole time-of-flight mass spectrometer. Anal. Chem. 82, 824–832 (2010).
Medvedev, A. E. et al. Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. J. Biol. Chem. 282, 16042–16053 (2007).
Rock, F. L., Hardiman, G., Timans, J. C., Kastelein, R. A. & Bazan, J. F. A family of human receptors structurally related to Drosophila Toll. Proc. Natl Acad. Sci. USA 95, 588–593 (1998).
Wang, P., Zhu, F., Tong, Z. & Konstantopoulos, K. Response of chondrocytes to shear stress: antagonistic effects of the binding partners Toll-like receptor 4 and caveolin-1. FASEB J. 25, 3401–3415 (2011).
Midwood, K. et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat. Med. 15, 774–780 (2009).
Kikuchi, T. et al. Gene expression of osteoclast differentiation factor is induced by lipopolysaccharide in mouse osteoblasts via Toll-like receptors. J. Immunol. 166, 3574–3579 (2001).
Stewart, C. R. et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 11, 155–161 (2010).
Sohn, D. H. et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res. Ther. 14, R7 (2012).
Liu-Bryan, R. & Terkeltaub, R. Chondrocyte innate immune myeloid differentiation factor 88-dependent signaling drives procatabolic effects of the endogenous Toll-like receptor 2/Toll-like receptor 4 ligands low molecular weight hyaluronan and high mobility group box chromosomal protein 1 in mice. Arthritis Rheum. 62, 2004–2012 (2010).
Yang, H. et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc. Natl Acad. Sci. USA 107, 11942–11947 (2010).
Frommer, K. W. et al. Free fatty acids: potential proinflammatory mediators in rheumatic diseases. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2013-203755.
Lee, J. Y. et al. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J. Biol. Chem. 279, 16971–16979 (2004).
Scott, P., Ma, H., Viriyakosol, S., Terkeltaub, R. & Liu-Bryan, R. Engagement of CD14 mediates the inflammatory potential of monosodium urate crystals. J. Immunol. 177, 6370–6378 (2006).
De Seny, D. et al. Acute-phase serum amyloid A in osteoarthritis: regulatory mechanism and proinflammatory properties. PLoS ONE 8, e66769 (2013).
Tsukamoto, H., Fukudome, K., Takao, S., Tsuneyoshi, N. & Kimoto, M. Lipopolysaccharide-binding protein-mediated Toll-like receptor 4 dimerization enables rapid signal transduction against lipopolysaccharide stimulation on membrane-associated CD14-expressing cells. Int. Immunol. 22, 271–280 (2010).
Haziot, A. et al. Resistance to endotoxin shock and reduced dissemination of Gram-negative bacteria in CD14-deficient mice. Immunity 4, 407–414 (1996).
Lien, E. et al. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J. Clin. Invest. 105, 497–504 (2000).
Poltorak, A., Ricciardi-Castagnoli, P., Citterio, S. & Beutler, B. Physical contact between lipopolysaccharide and Toll-like receptor 4 revealed by genetic complementation. Proc. Natl Acad. Sci. USA 97, 2163–2167 (2000).
Da Silva Correia, J. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. transfer from CD14 to TLR4 and MD-2. J. Biol. Chem. 276, 21129–21135 (2001).
Tsan, M.-F. & Gao, B. Pathogen-associated molecular pattern contamination as putative endogenous ligands of Toll-like receptors. J. Endotoxin Res. 13, 6–14 (2007).
Marincek, B.-C. et al. Heat shock protein-antigen fusions lose their enhanced immunostimulatory capacity after endotoxin depletion. Mol. Immunol. 46, 181–191 (2008).
Luong, M. et al. Stimulation of TLR4 by recombinant HSP70 requires structural integrity of the HSP70 protein itself. J. Inflamm (Lond.) 9, 11 (2012).
Okamura, Y. et al. The extra domain A of fibronectin activates Toll-like receptor 4. J. Biol. Chem. 276, 10229–10233 (2001).
Schelbergen, R. F. P. et al. Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on Toll-like receptor 4. Arthritis Rheum. 64, 1477–1487 (2012).
Akira, S. & Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 (2004).
Takeda, K. & Akira, S. TLR signaling pathways. Semin. Immunol. 16, 3–9 (2004).
Lin, S.-C., Lo, Y.-C. & Wu, H. Helical assembly in the MyD88–IRAK4–IRAK2 complex in TLR/IL-1R signalling. Nature 465, 885–890 (2010).
Motshwene, P. G. et al. An oligomeric signaling platform formed by the Toll-like receptor signal transducers MyD88 and IRAK-4. J. Biol. Chem. 284, 25404–25411 (2009).
Goldring, M. B. & Goldring, S. R. Osteoarthritis. J. Cell Physiol. 213, 626–634 (2007).
Kagan, J. C. et al. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-β. Nat. Immunol. 9, 361–368 (2008).
Tanimura, N., Saitoh, S., Matsumoto, F., Akashi-Takamura, S. & Miyake, K. Roles for LPS-dependent interaction and relocation of TLR4 and TRAM in TRIF-signaling. Biochem. Biophys. Res. Commun. 368, 94–99 (2008).
Bonham, K. S. et al. A promiscuous lipid-binding protein diversifies the subcellular sites of Toll-like receptor signal transduction. Cell 156, 705–716 (2014).
Sillat, T. et al. Toll-like receptors in human chondrocytes and osteoarthritic cartilage. Acta Orthop. 84, 585–592 (2013).
Karlsson, C. et al. Genome-wide expression profiling reveals new candidate genes associated with osteoarthritis. Osteoarthritis Cartilage 18, 581–592 (2010).
Kim, H. A. et al. The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. 54, 2152–2163 (2006).
Bobacz, K. et al. Toll-like receptors and chondrocytes: the lipopolysaccharide-induced decrease in cartilage matrix synthesis is dependent on the presence of Toll-like receptor 4 and antagonized by bone morphogenetic protein 7. Arthritis Rheum. 56, 1880–1893 (2007).
Abdollahi-Roodsaz, S. et al. Local interleukin-1-driven joint pathology is dependent on Toll-like receptor 4 activation. Am. J. Pathol. 175, 2004–2013 (2009).
Haglund, L., Bernier, S. M., Onnerfjord, P. & Recklies, A. D. Proteomic analysis of the LPS-induced stress response in rat chondrocytes reveals induction of innate immune response components in articular cartilage. Matrix Biol. 27, 107–118 (2008).
Verzijl, N. et al. Age-related accumulation of Maillard reaction products in human articular cartilage collagen. Biochem. J. 350 (Pt 2), 381–387 (2000).
Chen, Y. J., Sheu, M. L., Tsai, K. S., Yang, R. S. & Liu, S. H. Advanced glycation end products induce peroxisome proliferator-activated receptor γ down-regulation-related inflammatory signals in human chondrocytes via Toll-like receptor-4 and receptor for advanced glycation end products. PLoS ONE 8, e66611 (2013).
Hardy, M. M. et al. Cyclooxygenase 2-dependent prostaglandin E2 modulates cartilage proteoglycan degradation in human osteoarthritis explants. Arthritis Rheum. 46, 1789–1803 (2002).
Miller, C. et al. Transcriptional induction of cyclooxygenase-2 gene by okadaic acid inhibition of phosphatase activity in human chondrocytes: co-stimulation of AP-1 and CRE nuclear binding proteins. J. Cell Biochem. 69, 392–413 (1998).
Wang, P., Zhu, F. & Konstantopoulos, K. Interleukin-6 synthesis in human chondrocytes is regulated via the antagonistic actions of prostaglandin (PG)E2 and 15-deoxy-Δ12,14-PGJ2 . PLoS ONE 6, e27630 (2011).
Álvarez-Soria, M. A. et al. Long-term NSAID treatment directly decreases COX-2 and mPGES-1 production in the articular cartilage of patients with osteoarthritis. Osteoarthritis Cartilage 16, 1484–1493 (2008).
Abramson, S. B., Attur, M., Amin, A. R. & Clancy, R. Nitric oxide and inflammatory mediators in the perpetuation of osteoarthritis. Curr. Rheumatol. Rep. 3, 535–541 (2001).
Pelletier, J. P., Mineau, F., Ranger, P., Tardif, G. & Martel-Pelletier, J. The increased synthesis of inducible nitric oxide inhibits IL-1RA synthesis by human articular chondrocytes: possible role in osteoarthritic cartilage degradation. Osteoarthritis Cartilage 4, 77–84 (1996).
Stadler, J. et al. Articular chondrocytes synthesize nitric oxide in response to cytokines and lipopolysaccharide. J. Immunol. 147, 3915–3920 (1991).
Wu, G.-J. et al. Nitric oxide from both exogenous and endogenous sources activates mitochondria-dependent events and induces insults to human chondrocytes. J. Cell Biochem. 101, 1520–1531 (2007).
Gómez, R. et al. Nitric oxide boosts TLR-4 mediated lipocalin 2 expression in chondrocytes. J. Orthop. Res. 31, 1046–1052 (2013).
Conde, J. et al. Expanding the adipokine network in cartilage: identification and regulation of novel factors in human and murine chondrocytes. Ann. Rheum. Dis. 70, 551–559 (2011).
Ayral, X., Pickering, E. H., Woodworth, T. G., McKillop, N. & Dougados, M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis—results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage 13, 361–367 (2005).
Ospelt, C. et al. Overexpression of Toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: Toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum. 58, 3684–3692 (2008).
Jung, Y. O. et al. Synergism of Toll-like receptor 2 (TLR2), TLR4, and TLR6 ligation on the production of tumor necrosis factor (TNF)-α in a spontaneous arthritis animal model of interleukin (IL)-1 receptor antagonist-deficient mice. Immunol. Lett. 123, 138–143 (2009).
Sanchez, C. et al. Regulation of subchondral bone osteoblast metabolism by cyclic compression. Arthritis Rheum. 64, 1193–1203 (2011).
Chiba, K. et al. Osteoporotic changes of subchondral trabecular bone in osteoarthritis of the knee: a 3-T MRI study. Osteoporos. Int. 23, 589–597 (2012).
Zhang, L.-Z. et al. Mechanical and biologic link between cartilage and subchondral bone in osteoarthritis. Arthritis Care Res. 64, 960–967 (2012).
Zhen, G. & Cao, X. Targeting TGFβ signaling in subchondral bone and articular cartilage homeostasis. Trends Pharmacol. Sci. 35, 227–236 (2014).
Lacourt, M. et al. Relationship between cartilage and subchondral bone lesions in repetitive impact trauma-induced equine osteoarthritis. Osteoarthritis Cartilage 20, 572–583 (2012).
Zou, W., Amcheslavsky, A. & Bar-Shavit, Z. CpG oligodeoxynucleotides modulate the osteoclastogenic activity of osteoblasts via Toll-like receptor 9. J. Biol. Chem. 278, 16732–16740 (2003).
Nemoto, E., Honda, T., Kanaya, S., Takada, H. & Shimauchi, H. Expression of functional Toll-like receptors and nucleotide-binding oligomerization domain proteins in murine cementoblasts and their upregulation during cell differentiation. J. Periodont. Res. 43, 585–593 (2008).
Gao, A., Kantarci, A., Herrera, B. S., Gao, H. & Van Dyke T. E. A critical role for suppressors of cytokine signaling 3 in regulating LPS-induced transcriptional activation of matrix metalloproteinase-13 in osteoblasts. PeerJ 1, e51 (2013).
Inada, M., Matsumoto, C., Uematsu, S., Akira, S. & Miyaura, C. Membrane-bound prostaglandin E synthase-1-mediated prostaglandin E2 production by osteoblast plays a critical role in lipopolysaccharide-induced bone loss associated with inflammation. J. Immunol. 177, 1879–1885 (2006).
Nakao, J. et al. Low-intensity pulsed ultrasound (LIPUS) inhibits LPS-induced inflammatory responses of osteoblasts through TLR4–MyD88 dissociation. Bone 58, 17–25 (2013).
Bandow, K. et al. Molecular mechanisms of the inhibitory effect of lipopolysaccharide (LPS) on osteoblast differentiation. Biochem. Biophys. Res. Commun. 402, 755–761 (2010).
Huang, R.-L. et al. LPS-stimulated inflammatory environment inhibits BMP-2-induced osteoblastic differentiation through crosstalk between TLR4/MyD88/NF-κB and BMP/Smad signaling. Stem Cells Dev. 23, 277–289 (2014).
Raicevic, G. et al. Inflammation and Toll-like receptor ligation differentially affect the osteogenic potential of human mesenchymal stromal cells depending on their tissue origin. Tissue Eng. Part A 18, 1410–1418 (2012).
Kim, Y. S. et al. Increased circulating heat shock protein 60 induced by menopause, stimulates apoptosis of osteoblast-lineage cells via up-regulation of Toll-like receptors. Bone 45, 68–76 (2009).
Liu, J. et al. Molecular mechanism of the bifunctional role of lipopolysaccharide in osteoclastogenesis. J. Biol. Chem. 284, 12512–12523 (2009).
Itoh, K. et al. Lipopolysaccharide promotes the survival of osteoclasts via Toll-like receptor 4, but cytokine production of osteoclasts in response to lipopolysaccharide is different from that of macrophages. J. Immunol. 170, 3688–3695 (2003).
Clockaerts, S. et al. The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: a narrative review. Osteoarthritis Cartilage 18, 876–882 (2010).
Eymard, F. et al. Infrapatellar fat pad induces an inflammatory and a pro-degradative phenotype in autologous fibroblast-like synoviocytes from patients with knee OA. Arthritis Rheumatol. 66, 2165–2174 (2014).
Klein-Wieringa, I. R. et al. The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype. Ann. Rheum. Dis. 70, 851–857 (2011).
Distel, E. et al. The infrapatellar fat pad in knee osteoarthritis: an important source of interleukin-6 and its soluble receptor. Arthritis Rheum. 60, 3374–3377 (2009).
Bès-Houtmann, S. et al. Presence of functional TLR2 and TLR4 on human adipocytes. Histochem. Cell Biol. 127, 131–137 (2007).
Orr, J. S. et al. Toll-like receptor 4 deficiency promotes the alternative activation of adipose tissue macrophages. Diabetes 61, 2718–2727 (2012).
Davis, J. E., Gabler, N. K., Walker-Daniels, J. & Spurlock, M. E. TLR-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity (Silver Spring) 16, 1248–1255 (2008).
Jia, L. et al. Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nat. Commun. 5, 3878 (2014).
Campo, G. M. et al. Small hyaluronan oligosaccharides induce inflammation by engaging both Toll-like-4 and CD44 receptors in human chondrocytes. Biochem. Pharmacol. 80, 480–490 (2010).
Campo, G. M. et al. Hyaluronan differently modulates TLR-4 and the inflammatory response in mouse chondrocytes. Biofactors 38, 69–76 (2012).
Wähämaa, H. et al. High mobility group box protein 1 in complex with lipopolysaccharide or IL-1 promotes an increased inflammatory phenotype in synovial fibroblasts. Arthritis Res. Ther. 13, R136 (2011).
Sofat, N. Analysing the role of endogenous matrix molecules in the development of osteoarthritis. Int. J. Exp. Pathol. 90, 463–479 (2009).
Homandberg, G. A., Meyers, R. & Xie, D. L. Fibronectin fragments cause chondrolysis of bovine articular cartilage slices in culture. J. Biol. Chem. 267, 3597–3604 (1992).
Xie, D. L., Meyers, R. & Homandberg, G. A. Fibronectin fragments in osteoarthritic synovial fluid. J. Rheumatol. 19, 1448–1452 (1992).
Brown, R. A. & Jones, K. L. The synthesis and accumulation of fibronectin by human articular cartilage. J. Rheumatol. 17, 65–72 (1990).
Zhen, E. Y. et al. Characterization of metalloprotease cleavage products of human articular cartilage. Arthritis Rheum. 58, 2420–2431 (2008).
Sofat, N. Robertson, S. D. & Wait, R. Fibronectin III 13–14 domains induce joint damage via Toll-like receptor 4 activation and synergize with interleukin-1 and tumour necrosis factor. J. Innate Immun. 4, 69–79 (2011).
Zreiqat, H. et al. S100A8 and S100A9 in experimental osteoarthritis. Arthritis Res. Ther. 12, R16 (2010).
Kalichman, L. & Kobyliansky, E. Hand osteoarthritis in Chuvashian population: prevalence and determinants. Rheumatol. Int. 30, 85–92 (2009).
Villalvilla, A., Gómez, R., Largo, R. & Herrero-Beaumont, G. Lipid transport and metabolism in healthy and osteoarthritic cartilage. Int. J. Mol. Sci. 14, 20793–20808 (2013).
Cillero-Pastor, B., Eijkel, G., Kiss, A., Blanco, F. J. & Heeren, R. M. Time-of-flight secondary ion mass spectrometry-based molecular distribution distinguishing healthy and osteoarthritic human cartilage. Anal. Chem. 84, 8909–8916 (2012).
Alvarez-Garcia, O., Rogers, N. H., Smith, R. G. & Lotz, M. K. Palmitate has proapoptotic and proinflammatory effects on articular cartilage and synergizes with interleukin-1. Arthritis Rheumatol. 66, 1779–1788 (2014).
Moisio, K. et al. Denuded subchondral bone and knee pain in persons with knee osteoarthritis. Arthritis Rheum. 60, 3703–3710 (2009).
Ballegaard, C. et al. Knee pain and inflammation in the infrapatellar fat pad estimated by conventional and dynamic contrast-enhanced magnetic resonance imaging in obese patients with osteoarthritis: a cross-sectional study. Osteoarthritis Cartilage 22, 933–940 (2014).
Hill, C. L. et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann. Rheum. Dis. 66, 1599–1603 (2007).
Liu, T., Gao, Y.-J. & Ji, R.-R. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci. Bull. 28, 131–144 (2012).
Tanga, F. Y., Nutile-McMenemy, N. & DeLeo, J. A. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc. Natl Acad. Sci. USA 102, 5856–5861 (2005).
Campo, G. M. et al. Adenosine A2A receptor activation and hyaluronan fragment inhibition reduce inflammation in mouse articular chondrocytes stimulated with interleukin-1β. FEBS J. 279, 2120–2133 (2012).
Chang, E.-J. et al. Hyaluronan inhibits osteoclast differentiation via Toll-like receptor 4. J. Cell Sci. 120, 166–176 (2007).
Vasheghani, F. et al. Adult cartilage-specific peroxisome proliferator-activated receptor γ knockout mice exhibit the spontaneous osteoarthritis phenotype. Am. J. Pathol. 182, 1099–1106 (2013).
Juarranz, Y. et al. VIP decreases TLR4 expression induced by LPS and TNF-α treatment in human synovial fibroblasts. Ann. NY Acad. Sci. 1070, 359–364 (2006).
Haskó, G., Linden, J., Cronstein, B. & Pacher, P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 7, 759–770 (2008).
Campo, G. M. et al. The stimulation of adenosine 2A receptor reduces inflammatory response in mouse articular chondrocytes treated with hyaluronan oligosaccharides. Matrix Biology 31, 338–351 (2012).
Peri, F. & Piazza, M. Therapeutic targeting of innate immunity with Toll-like receptor 4 (TLR4) antagonists. Biotechnol. Adv. 30, 251–260 (2012).
Iacono, A. et al. Effect of oleocanthal and its derivatives on inflammatory response induced by lipopolysaccharide in a murine chondrocyte cell line. Arthritis Rheum. 62, 1675–1682 (2010).
Chen, Y. J. et al. EGb761 inhibits inflammatory responses in human chondrocytes and shows chondroprotection in osteoarthritic rat knee. J. Orthop. Res. 31, 1032–1038 (2013).
Villalvilla, A. et al. 6-Shogaol inhibits chondrocytes' innate immune responses and cathepsin-K activity. Mol. Nutr. Food Res. 58, 256–266 (2014).
Ahn, S.-I., Lee, J.-K. & Youn, H.-S. Inhibition of homodimerization of Toll-like receptor 4 by 6-shogaol. Mol. Cells 27, 211–215 (2009).
Wang, Q. et al. Oral and topical boswellic acid attenuates mouse osteoarthritis. Osteoarthritis Cartilage 22, 128–132 (2014).
Barochia, A., Solomon, S., Cui, X., Natanson, C. & Eichacker, P. Q. Eritoran tetrasodium (E5564) treatment for sepsis: review of preclinical and clinical studies. Expert Opin. Drug Metab. Toxicol. 7, 479–494 (2011).
Shirey, K. A. et al. The TLR4 antagonist eritoran protects mice from lethal influenza infection. Nature 497, 498–502 (2013).
Neal, M. D. et al. Discovery and validation of a new class of small molecule Toll-like receptor 4 (TLR4) inhibitors. PLoS ONE 8, e65779 (2013).
Shang, L. et al. Selective antibody intervention of Toll-like receptor 4 activation through Fc γ receptor tethering. J. Biol. Chem. 289, 15309–15318 (2014).
Piao, W., Vogel, S. N. & Toshchakov, V. Y. Inhibition of TLR4 signaling by TRAM-derived decoy peptides in vitro and in vivo. J. Immunol. 190, 2263–2272 (2013).
Hutchinson, M. R. et al. Evidence that tricyclic small molecules may possess Toll-like receptor and myeloid differentiation protein 2 activity. Neuroscience 168, 551–563 (2010).
Hutchinson, M. R. et al. Evidence that opioids may have Toll-like receptor 4 and MD-2 effects. Brain Behav. Immun. 24, 83–95 (2010).
Piazza, M. et al. Hemin and a metabolic derivative coprohemin modulate the TLR4 pathway differently through different molecular targets. Innate Immun. 17, 293–301 (2011).
Noman, A. S. M. et al. Thalidomide inhibits lipopolysaccharide-induced tumor necrosis factor-α production via down-regulation of MyD88 expression. Innate Immun. 15, 33–41 (2009).
Chevalier, X. et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 61, 344–352 (2009).
Bellido, M. et al. Improving subchondral bone integrity reduces progression of cartilage damage in experimental osteoarthritis preceded by osteoporosis. Osteoarthritis Cartilage 19, 1228–1236 (2011).
WHO. Chronic diseases and health promotion. Chronic rheumatic conditions [online], (2014).
Wan, Y. PPARγ in bone homeostasis. Trends Endocrinol. Metab. 21, 722–728 (2010).
Gosset, P. et al. Prostaglandin D2 affects the differentiation and functions of human dendritic cells: impact on the T cell response. Eur. J. Immunol. 35, 1491–1500 (2005).
Arima, M. & Fukuda, T. Prostaglandin D2 receptors DP and CRTH2 in the pathogenesis of asthma. Curr. Mol. Med. 8, 365–375 (2008).
Schindler, C. W. et al. Role of central and peripheral adenosine receptors in the cardiovascular responses to intraperitoneal injections of adenosine A1 and A2A subtype receptor agonists. Br. J. Pharmacol. 144, 642–650 (2005).
Acknowledgements
The authors' research is supported by research grants from Fondo de Investigación Sanitaria (PI12/00144 and PI13/00570). R.G. was funded by the Instituto de Salud Carlos III through a Sara Borrell programme. A.V. is the recipient of a fellowship from the Fundación Conchita Rábago. R.L. and O.G. were funded by the Instituto de Salud Carlos III through a research staff stabilization programme.
Author information
Authors and Affiliations
Contributions
R.G. researched data for the article and wrote the manuscript. R.G., A.V., R.L., O.G. and G.H.-B. substantially contributed to discussion of content and reviewing and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Gómez, R., Villalvilla, A., Largo, R. et al. TLR4 signalling in osteoarthritis—finding targets for candidate DMOADs. Nat Rev Rheumatol 11, 159–170 (2015). https://doi.org/10.1038/nrrheum.2014.209
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2014.209
This article is cited by
-
Resatorvid alleviates experimental inflammatory TMJOA by restraining chondrocyte pyroptosis and synovial inflammation
Arthritis Research & Therapy (2023)
-
4,8-Dicarboxyl-8,9-iridoid-1-glycoside inhibits apoptosis in human osteoarthritis chondrocytes via enhanced c-MYC-mediated cholesterol metabolism in vitro
Arthritis Research & Therapy (2023)
-
Toll-like receptor 4 (TLR4): new insight immune and aging
Immunity & Ageing (2023)
-
The Potential Role of Probiotics in the Management of Osteoarthritis Pain: Current Status and Future Prospects
Current Rheumatology Reports (2023)
-
TLR4 downregulation by the RNA-binding protein PUM1 alleviates cellular aging and osteoarthritis
Cell Death & Differentiation (2022)