Key Points
-
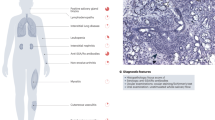
Treatment decisions in primary Sjögren syndrome (pSS) are based on the initial evaluation of symptoms and extraglandular manifestations
-
Sicca is managed by education and environment modification, elimination of contingent offending drugs, artificial tears, secretagogues and treatments for complications
-
Severe and acute systemic manifestations require treatment with glucocorticoids and/or immunosuppressant drugs
-
The role for biologic therapies is controversial as no double-blind randomized controlled trials (RCTs) establishing their efficacy are available
-
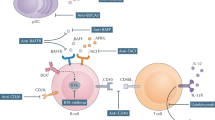
Targets for new treatments directed against the immunopathological mechanisms of pSS have been identified
-
Patients who fail to respond to conventional treatment should be invited to participate in ongoing RCTs of novel therapies
Abstract
Primary Sjögren syndrome (pSS) is a progressive autoimmune disease characterized by sicca and systemic manifestations. In this Review, we summarize the available data on topical and systemic medications, according to clinical signs and disease activity, and we describe the ongoing studies using biologic drugs in the treatment of pSS. Expanding knowledge about the epidemiology, classification criteria, systemic activity scoring (ESSDAI) and patient-reported outcomes (ESSPRI) is driving active research. Treatment decisions are based on the evaluation of symptoms and extraglandular manifestations. Symptomatic treatment is usually appropriate, whereas systemic treatment is reserved for systemic manifestations. Sicca is managed by education, environment modification, elimination of contingent offending drugs, artificial tears, secretagogues and treatments for complications. Mild systemic signs such as fatigue are treated by exercise. Pain can require short-term moderate-dose glucocorticoid therapy and, in some cases, disease-modifying drugs. Severe and acute systemic manifestations indicate treatment with glucocorticoids and/or immunosuppressant drugs. The role for biologic agents is promising, but no double-blind randomized controlled trials (RCTs) proving the efficacy of these drugs are available. Targets for new treatments directed against the immunopathological mechanisms of pSS include epithelial cells, T cells, B-cell overactivity, the interferon signature, proinflammatory cytokines, ectopic germinal centre formation, chemokines involved in lymphoid cell homing, and epigenetic modifications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Fox, R. I. Sjögren's syndrome. Lancet 366, 321–331 (2005).
Cornec, D. et al. The differential diagnosis of dry eyes, dry mouth, and parotidomegaly: a comprehensive review. Clin. Rev. Allergy Immunol. 49, 278–287 (2015).
Pijpe, J. et al. Progression of salivary gland dysfunction in patients with Sjögren's syndrome. Ann. Rheum. Dis. 66, 107–112 (2007).
Vitali, C. et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 61, 554–558 (2002).
Shiboski, S. C. et al. American College of Rheumatology classification criteria for Sjögren's syndrome: a data-driven, expert consensus approach in the Sjögren's International Collaborative Clinical Alliance cohort. Arthritis Care Res. (Hoboken) 64, 475–487 (2012).
Shiboski, C. H. & Shiboski, S. C. Proposed ACR–EULAR classification criteria for Sjögren's syndrome: development and validation [abstract S1.1]. Scand. J. Immunol. 81, 330 (2015).
Seror, R. et al. EULAR Sjögren's syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjögren's syndrome. Ann. Rheum. Dis. 69, 1103–1109 (2010).
Seror, R. et al. Defining disease activity states and clinically meaningful improvement in primary Sjogren's syndrome with EULAR primary Sjögren's syndrome disease activity (ESSDAI) and patient-reported indexes (ESSPRI). Ann. Rheum. Dis. 75, 382–389 (2016).
Seror, R. et al. EULAR Sjögren's Syndrome Patient Reported Index (ESSPRI): development of a consensus patient index for primary Sjögren's syndrome. Ann. Rheum. Dis. 70, 968–972 (2011).
Theander, E. et al. Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjögren's syndrome. Ann. Rheum. Dis. 70, 1363–1368 (2011).
Voulgarelis, M. et al. Prognosis and outcome of non-Hodgkin lymphoma in primary Sjögren syndrome. Med. (Baltimore) 91, 1–9 (2012).
Baimpa, E., Dahabreh, I. J., Voulgarelis, M. & Moutsopoulos, H. M. Hematologic manifestations and predictors of lymphoma development in primary Sjögren syndrome: clinical and pathophysiologic aspects. Med. (Baltimore) 88, 284–293 (2009).
Tobon, G. J. et al. Role of Fms-like tyrosine kinase 3 ligand as a potential biologic marker of lymphoma in primary Sjögren's syndrome. Arthritis Rheum. 65, 3218–3227 (2013).
Papageorgiou, A. et al. A BAFF receptor His159Tyr mutation in Sjögren's syndrome-related lymphoproliferation. Arthritis Rheumatol. 67, 2732–2741 (2015).
Valim, V. et al. Recommendations for the treatment of Sjögren's syndrome. Rev. Bras. Reumatol. 55, 446–457 (in Portuguese) (2015).
Furness, S., Worthington, H. V., Bryan, G., Birchenough, S. & McMillan, R. Interventions for the management of dry mouth: topical therapies. Cochrane Database Syst. Rev. 12, CD008934 (2011).
Akpek, E. K. et al. Treatment of Sjögren's syndrome-associated dry eye an evidence-based review. Ophthalmology 118, 1242–1252 (2011).
Ramos-Casals, M., Tzioufas, A. G., Stone, J. H., Siso, A. & Bosch, X. Treatment of primary Sjögren syndrome: a systematic review. JAMA 304, 452–460 (2010).
Foulks, G. N. et al. Clinical guidelines for management of dry eye associated with Sjögren disease. Ocul. Surf. 13, 118–132 (2015).
Amerongen, A. V. & Veerman, E. C. Saliva — the defender of the oral cavity. Oral Dis. 8, 12–22 (2002).
Dawes, C. et al. The functions of human saliva: a review sponsored by the World Workshop on Oral Medicine VI. Arch. Oral Biol. 60, 863–874 (2015).
Mavragani, C. P. & Moutsopoulos, H. M. Conventional therapy of Sjögren's syndrome. Clin. Rev. Allergy Immunol. 32, 284–291 (2007).
Najera, M. P. et al. Prevalence of periodontal disease in patients with Sjögren's syndrome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 83, 453–457 (1997).
Boutsi, E. A., Paikos, S., Dafni, U. G., Moutsopoulos, H. M. & Skopouli, F. N. Dental and periodontal status of Sjögren's syndrome. J. Clin. Periodontol. 27, 231–235 (2000).
Lugonja, B. et al. Periodontitis prevalence and serum antibody reactivity to periodontal bacteria in primary Sjögren's syndrome: a pilot study. J. Clin. Periodontol. 43, 26–33 (2016).
Le Gall, M. et al. A prospective evaluation of dental and periodontal status in patients with suspected Sjögren's syndrome. Joint Bone Spine 83, 235–236 (2016).
van der Reijden, W. A., van der Kwaak, H., Vissink, A., Veerman, E. C. & Amerongen, A. V. Treatment of xerostomia with polymer-based saliva substitutes in patients with Sjögren's syndrome. Arthritis Rheum. 39, 57–63 (1996).
Bartold, P. M. Dentinal hypersensitivity: a review. Aust. Dent. J. 51, 212–218 (2006).
Plemons, J. M., Al-Hashimi, I. & Marek, C. L. and American Dental Association Council on Scientific Affairs. Managing xerostomia and salivary gland hypofunction: executive summary of a report from the American Dental Association Council on Scientific Affairs. J. Am. Dent. Assoc. 145, 867–873 (2014).
Steller, M., Chou, L. & Daniels, T. E. Electrical stimulation of salivary flow in patients with Sjögren's syndrome. J. Dent. Res. 67, 1334–1337 (1988).
Talal, N., Quinn, J. H. & Daniels, T. E. The clinical effects of electrostimulation on salivary function of Sjögren's syndrome patients. A placebo controlled study. Rheumatol. Int. 12, 43–45 (1992).
Strietzel, F. P. et al. Efficacy and safety of an intraoral electrostimulation device for xerostomia relief: a multicenter, randomized trial. Arthritis Rheum. 63, 180–190 (2011).
Vivino, F. B. et al. Pilocarpine tablets for the treatment of dry mouth and dry eye symptoms in patients with Sjögren syndrome: a randomized, placebo-controlled, fixed-dose, multicenter trial. P92-01 Study Group. Arch. Intern. Med. 159, 174–181 (1999).
Papas, A. S. et al. Successful treatment of dry mouth and dry eye symptoms in Sjögren's syndrome patients with oral pilocarpine: a randomized, placebo-controlled, dose-adjustment study. J. Clin. Rheumatol. 10, 169–177 (2004).
Wu, C. H. et al. Pilocarpine hydrochloride for the treatment of xerostomia in patients with Sjögren's syndrome in Taiwan — a double-blind, placebo-controlled trial. J. Formos. Med. Assoc. 105, 796–803 (2006).
Petrone, D. et al. A double-blind, randomized, placebo-controlled study of cevimeline in Sjögren's syndrome patients with xerostomia and keratoconjunctivitis sicca. Arthritis Rheum. 46, 748–754 (2002).
Fife, R. S. et al. Cevimeline for the treatment of xerostomia in patients with Sjögren syndrome: a randomized trial. Arch. Intern. Med. 162, 1293–1300 (2002).
Leung, K. C. et al. The efficacy of cevimeline hydrochloride in the treatment of xerostomia in Sjögren's syndrome in southern Chinese patients: a randomised double-blind, placebo-controlled crossover study. Clin. Rheumatol. 27, 429–436 (2008).
Meijer, J. M. et al. Effectiveness of rituximab treatment in primary Sjögren's syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 62, 960–968 (2010).
Dass, S. et al. Reduction of fatigue in Sjögren syndrome with rituximab: results of a randomised, double-blind, placebo-controlled pilot study. Ann. Rheum. Dis. 67, 1541–1544 (2008).
Devauchelle-Pensec, V. et al. Treatment of primary Sjögren syndrome with rituximab: a randomized trial. Ann. Intern. Med. 160, 233–242 (2014).
Forstot, S. L. & Foulks, G. N. Management of Dry Eye (Oxford Univ. Press, 2012).
Aragona, P. et al. Effects of amino acids enriched tears substitutes on the cornea of patients with dysfunctional tear syndrome. Acta Ophthalmol. 91, e437–e444 (2013).
Condon, P. I. et al. Double blind, randomised, placebo controlled, crossover, multicentre study to determine the efficacy of a 0.1% (w/v) sodium hyaluronate solution (Fermavisc) in the treatment of dry eye syndrome. Br. J. Ophtalmol. 83, 1121–1124 (1999).
Toda, I., Shinozaki, N. & Tsubota, K. Hydroxypropyl methylcellulose for the treatment of severe dry eye associated with Sjögren's syndrome. Cornea 15, 120–128 (1996).
Marsh, P. & Pflugfelder, S. C. Topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjögren syndrome. Ophthalmology 106, 811–816 (1999).
Barber, L. D., Pflugfelder, S. C., Tauber, J. & Foulks, G. N. Phase III safety evaluation of cyclosporine 0.1% ophthalmic emulsion administered twice daily to dry eye disease patients for up to 3 years. Ophthalmology 112, 1790–1794 (2005).
Sall, K., Stevenson, O. D., Mundorf, T. K. & Reis, B. L. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. Ophthalmology 107, 631–639 (2000).
Stevenson, D., Tauber, J. & Reis, B. L. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. Ophthalmology 107, 967–974 (2000).
Tsifetaki, N. et al. Oral pilocarpine for the treatment of ocular symptoms in patients with Sjögren's syndrome: a randomised 12 week controlled study. Ann. Rheum. Dis. 62, 1204–1207 (2003).
Milin, M. et al. Sicca symptoms are associated with similar fatigue, anxiety, depression, and quality-of-life impairments in patients with and without primary Sjögren's syndrome. Joint Bone Spine http://dx.doi.org/10.1016/j.jbspin.2015.10.005 (2016).
van Leeuwen, N. et al. Psychological profiles in patients with Sjögren's syndrome related to fatigue: a cluster analysis. Rheumatology (Oxford) 54, 776–783 (2015).
Segal, B. et al. Prevalence, severity, and predictors of fatigue in subjects with primary Sjögren's syndrome. Arthritis Rheum. 59, 1780–1787 (2008).
Gottenberg, J. E. et al. Effects of hydroxychloroquine on symptomatic improvement in primary Sjögren syndrome: the JOQUER randomized clinical trial. JAMA 312, 249–258 (2014).
Hartkamp, A. et al. Effect of dehydroepiandrosterone administration on fatigue, well-being, and functioning in women with primary Sjögren syndrome: a randomised controlled trial. Ann. Rheum. Dis. 67, 91–97 (2008).
Theander, E., Horrobin, D. F., Jacobsson, L. T. & Manthorpe, R. Gammalinolenic acid treatment of fatigue associated with primary Sjögren's syndrome. Scand. J. Rheumatol. 31, 72–79 (2002).
Virkki, L. M. et al. Dehydroepiandrosterone (DHEA) substitution treatment for severe fatigue in DHEA-deficient patients with primary Sjögren's syndrome. Arthritis Care Res. (Hoboken) 62, 118–124 (2010).
Kruize, A. A. et al. Hydroxychloroquine treatment for primary Sjögren's syndrome: a two year double blind crossover trial. Ann. Rheum. Dis. 52, 360–364 (1993).
Mariette, X. et al. Inefficacy of infliximab in primary Sjögren's syndrome: results of the randomized, controlled Trial of Remicade in Primary Sjögren's Syndrome (TRIPSS). Arthritis Rheum. 50, 1270–1276 (2004).
Sankar, V. et al. Etanercept in Sjögren's syndrome: a twelve-week randomized, double-blind, placebo-controlled pilot clinical trial. Arthritis Rheum. 50, 2240–2245 (2004).
Mariette, X. et al. Efficacy and safety of belimumab in primary Sjögren's syndrome: results of the BELISS open-label phase II study. Ann. Rheum. Dis. 74, 526–531 (2015).
Meiners, P. M. et al. Abatacept treatment reduces disease activity in early primary Sjögren's syndrome (open-label proof of concept ASAP study). Ann. Rheum. Dis. 73, 1393–1396 (2014).
Brown, S. et al. The TRACTISS Protocol: a randomised double blind placebo controlled clinical TRial of Anti-B-Cell Therapy In patients with primary Sjögren's Syndrome. BMC Musculoskelet. Disord. 15, 21 (2014).
Bowman, S. et al. Preliminary results of a double-blind randomised trial of rituximab anti-B-cell therapy in patients with primary Sjögrens syndrome [abstract 11L]. Arthritis Rheum. 67 (Suppl. 10) (2015).
Rice, D. H. Chronic inflammatory disorders of the salivary glands. Otolaryngol. Clin. North Am. 32, 813–818 (1999).
Koch, M., Iro, H. & Zenk, J. Stenosis and other non-sialolithiasis-related obstructions of the major salivary gland ducts. Modern treatment concepts. HNO 58, 218–224 (in German) (2010).
De Luca, R., Trodella, M., Vicidomini, A., Colella, G. & Tartaro, G. Endoscopic management of salivary gland obstructive diseases in patients with Sjögren's syndrome. J. Craniomaxillofac. Surg. 43, 1643–1649 (2015).
Jager, D. J., Karagozoglu, K. H., Maarse, F., Brand, H. S. & Forouzanfar, T. Sialendoscopy of salivary glands affected by Sjögren syndrome: a randomized controlled pilot study. J. Oral Maxillofac. Surg. 74, 1167–1174 (2016).
Madero-Visbal, R. & Milas, Z. The role of parotidectomy in Sjögren's syndrome. Oral Maxillofac. Surg. Clin. North Am. 26, 83–90 (2014).
De Vita, S. et al. Efficacy and safety of belimumab given for 12 months in primary Sjögren's syndrome: the BELISS open-label phase II study. Rheumatology (Oxford) (2015).
Vitali, C. & Del Papa, N. Pain in primary Sjögren's syndrome. Best Pract. Res. Clin. Rheumatol. 29, 63–70 (2015).
Fauchais, A. L. et al. Articular manifestations in primary Sjögren's syndrome: clinical significance and prognosis of 188 patients. Rheumatology (Oxford) 49, 1164–1172 (2010).
Fox, R. I. et al. Treatment of primary Sjögren's syndrome with hydroxychloroquine. Am. J. Med. 85, 62–67 (1988).
Fox, R. I., Dixon, R., Guarrasi, V. & Krubel, S. Treatment of primary Sjögren's syndrome with hydroxychloroquine: a retrospective, open-label study. Lupus 5, S31–S36 (1996).
Sanders, S. & Harisdangkul, V. Leflunomide for the treatment of rheumatoid arthritis and autoimmunity. Am. J. Med. Sci. 323, 190–193 (2002).
Brito-Zeron, P. et al. Annular erythema in primary Sjögren's syndrome: description of 43 non-Asian cases. Lupus 23, 166–175 (2014).
Jessop, S., Whitelaw, D. A. & Delamere, F. M. Drugs for discoid lupus erythematosus. Cochrane Database Syst. Rev. 4, CD002954 (2009).
Kuhn, A., Ruland, V. & Bonsmann, G. Cutaneous lupus erythematosus: update of therapeutic options part I. J. Am. Acad. Dermatol. 65, e179–e193 (2011).
Ruzicka, T., Sommerburg, C., Goerz, G., Kind, P. & Mensing, H. Treatment of cutaneous lupus erythematosus with acitretin and hydroxychloroquine. Br. J. Dermatol. 127, 513–518 (1992).
Kuhn, A. et al. Influence of smoking on disease severity and antimalarial therapy in cutaneous lupus erythematosus: analysis of 1002 patients from the EUSCLE database. Br. J. Dermatol. 171, 571–579 (2014).
Ramos-Casals, M. et al. Primary Sjögren syndrome in Spain: clinical and immunologic expression in 1010 patients. Med. (Baltimore) 87, 210–219 (2008).
Palm, O. et al. Clinical pulmonary involvement in primary Sjögren's syndrome: prevalence, quality of life and mortality — a retrospective study based on registry data. Rheumatology (Oxford) 52, 173–179 (2013).
Gutsche, M., Rosen, G. D. & Swigris, J. J. Connective tissue disease-associated interstitial lung disease: a review. Curr. Respir. Care Rep. 1, 224–232 (2012).
Justet, A., Ottaviani, S., Dieude, P. & Taille, C. Tocilizumab for refractory organising pneumonia associated with Sjögren's disease.BMJ Case Rep. http://dx.doi.org/10.1136/bcr-2014-209076 (2015).
King, T. E. Jr. et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 370, 2083–2092 (2014).
Richeldi, L. et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 370, 2071–2082 (2014).
Goules, A. V., Tatouli, I. P., Moutsopoulos, H. M. & Tzioufas, A. G. Clinically significant renal involvement in primary Sjögren's syndrome: clinical presentation and outcome. Arthritis Rheum. 65, 2945–2953 (2013).
Francois, H. & Mariette, X. Renal involvement in primary Sjögren syndrome. Nat. Rev. Nephrol. 12, 82–93 (2016).
Maripuri, S. et al. Renal involvement in primary Sjögren's syndrome: a clinicopathologic study. Clin. J. Am. Soc. Nephrol. 4, 1423–1431 (2009).
Evans, R. D., Laing, C. M., Ciurtin, C. & Walsh, S. B. Tubulointerstitial nephritis in primary Sjögren syndrome: clinical manifestations and response to treatment. BMC Musculoskelet. Disord. 17, 2 (2016).
Rovin, B. H. et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 64, 1215–1226 (2012).
Arends, S. et al. Long-term follow-up of a randomised controlled trial of azathioprine/methylprednisolone versus cyclophosphamide in patients with proliferative lupus nephritis. Ann. Rheum. Dis. 71, 966–973 (2012).
Felson, D. T. & Anderson, J. Evidence for the superiority of immunosuppressive drugs and prednisone over prednisone alone in lupus nephritis. Results of a pooled analysis. N. Engl. J. Med. 311, 1528–1533 (1984).
Sfikakis, P. P. et al. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: an open-label trial. Arthritis Rheum. 52, 501–513 (2005).
Gunnarsson, I. et al. Histopathologic and clinical outcome of rituximab treatment in patients with cyclophosphamide-resistant proliferative lupus nephritis. Arthritis Rheum. 56, 1263–1272 (2007).
Gottenberg, J. E. et al. Efficacy of rituximab in systemic manifestations of primary Sjögren's syndrome: results in 78 patients of the AutoImmune and Rituximab registry. Ann. Rheum. Dis. 72, 1026–1031 (2013).
Atzeni, F. et al. Chronic widespread pain in the spectrum of rheumatological diseases. Best Pract. Res. Clin. Rheumatol. 25, 165–171 (2011).
Ruperto, N. et al. Prednisone versus prednisone plus ciclosporin versus prednisone plus methotrexate in new-onset juvenile dermatomyositis: a randomised trial. Lancet 387, 671–678 (2016).
Bunch, T. W., Worthington, J. W., Combs, J. J., Ilstrup, D. M. & Engel, A. G. Azathioprine with prednisone for polymyositis. A controlled, clinical trial. Ann. Intern. Med. 92, 365–369 (1980).
Majithia, V. & Harisdangkul, V. Mycophenolate mofetil (CellCept): an alternative therapy for autoimmune inflammatory myopathy. Rheumatology (Oxford) 44, 386–389 (2005).
Colafrancesco, S. et al. Myositis in primary Sjögren's syndrome: data from a multicentre cohort. Clin. Exp. Rheumatol. 33, 457–464 (2015).
Oddis, C. V. et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 65, 314–324 (2013).
Mok, C. C., Ho, L. Y. & To, C. H. Rituximab for refractory polymyositis: an open-label prospective study. J. Rheumatol. 34, 1864–1868 (2007).
Carvajal Alegria, G. et al. Epidemiology of neurological manifestations in Sjögren's syndrome: data from the French ASSESS Cohort. RMD Open 2, e000179 (2016).
Tobon, G. J., Pers, J. O., Devauchelle-Pensec, V. & Youinou, P. Neurological disorders in primary Sjögren's syndrome. Autoimmune Dis. 2012, 645967 (2012).
Nobile-Orazio, E. et al. Intravenous immunoglobulin versus intravenous methylprednisolone for chronic inflammatory demyelinating polyradiculoneuropathy: a randomised controlled trial. Lancet Neurol. 11, 493–502 (2012).
van Schaik, I. N. et al. Pulsed high-dose dexamethasone versus standard prednisolone treatment for chronic inflammatory demyelinating polyradiculoneuropathy (PREDICT study): a double-blind, randomised, controlled trial. Lancet Neurol. 9, 245–253 (2010).
Yamashita, H. et al. Diagnosis and treatment of primary Sjögren syndrome-associated peripheral neuropathy: a six-case series. Mod. Rheumatol. 23, 925–933 (2013).
Rist, S. et al. Experience of intravenous immunoglobulin therapy in neuropathy associated with primary Sjögren's syndrome: a national multicentric retrospective study. Arthritis Care Res. (Hoboken) 63, 1339–1344 (2011).
Martinez, A. R., Nunes, M. B., Nucci, A. & Franca, M. C. Jr Sensory neuronopathy and autoimmune diseases. Autoimmune Dis. 2012, 873587 (2012).
Chen, W. H., Yeh, J. H. & Chiu, H. C. Plasmapheresis in the treatment of ataxic sensory neuropathy associated with Sjögren's syndrome. Eur. Neurol. 45, 270–274 (2001).
Mekinian, A. et al. Efficacy of rituximab in primary Sjögren's syndrome with peripheral nervous system involvement: results from the AIR registry. Ann. Rheum. Dis. 71, 84–87 (2012).
Misery, L., Pavy-Le Traon, A., Genestet, S., Le Bec, R. & Marcorelles, P. Diagnosis of small-fibre neuropathies: comparison between quantitative sensory testing and the measurement of intraepidermal nerve fibre density. J. Eur. Acad. Dermatol. Venereol. 28, 825–826 (2014).
Devigili, G. et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 131, 1912–1925 (2008).
Fauchais, A. L. et al. Small fibre neuropathy in primary Sjögren syndrome. Rev. Med. Interne 32, 142–148 (in French) (2011).
Flanagan, E. P. et al. Short myelitis lesions in aquaporin-4-IgG-positive neuromyelitis optica spectrum disorders. JAMA Neurol. 72, 81–87 (2015).
Radaelli, M. et al. Neuromyelitis optica spectrum disorders: long-term safety and efficacy of rituximab in Caucasian patients. Mult. Scler. 22, 511–519 (2016).
Mealy, M. A., Wingerchuk, D. M., Palace, J., Greenberg, B. M. & Levy, M. Comparison of relapse and treatment failure rates among patients with neuromyelitis optica: multicenter study of treatment efficacy. JAMA Neurol. 71, 324–330 (2014).
Trebst, C. et al. Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the Neuromyelitis Optica Study Group (NEMOS). J. Neurol. 261, 1–16 (2014).
Cacoub, P., Comarmond, C., Domont, F., Savey, L. & Saadoun, D. Cryoglobulinemia vasculitis. Am. J. Med. 128, 950–955 (2015).
Terrier, B. et al. Non HCV-related infectious cryoglobulinemia vasculitis: results from the French nationwide CryoVas survey and systematic review of the literature. J. Autoimmun. 65, 74–81 (2015).
Visentini, M. et al. Efficacy of low-dose rituximab for the treatment of mixed cryoglobulinemia vasculitis: phase II clinical trial and systematic review. Autoimmun. Rev. 14, 889–896 (2015).
De Vita, S. et al. A randomized controlled trial of rituximab for the treatment of severe cryoglobulinemic vasculitis. Arthritis Rheum. 64, 843–853 (2012).
Singh, A. G., Singh, S. & Matteson, E. L. Rate, risk factors and causes of mortality in patients with Sjögren's syndrome: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford) 55, 450–460 (2016).
Johnsen, S. J. et al. Risk of non-Hodgkin's lymphoma in primary Sjögren's syndrome: a population-based study. Arthritis Care Res. (Hoboken) 65, 816–821 (2013).
Nocturne, G. & Mariette, X. Sjögren Syndrome-associated lymphomas: an update on pathogenesis and management. Br. J. Haematol. 168, 317–327 (2015).
Pollard, R. P. et al. Treatment of mucosa-associated lymphoid tissue lymphoma in Sjögren's syndrome: a retrospective clinical study. J. Rheumatol. 38, 2198–2208 (2011).
Papageorgiou, A. et al. Predicting the outcome of Sjögren's syndrome-associated non-hodgkin's lymphoma patients. PLoS ONE 10, e0116189 (2015).
Rummel, M. J. et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 381, 1203–1210 (2013).
Voulgarelis, M., Giannouli, S., Tzioufas, A. G. & Moutsopoulos, H. M. Long term remission of Sjögren's syndrome associated aggressive B cell non-Hodgkin's lymphomas following combined B cell depletion therapy and CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone). Ann. Rheum. Dis. 65, 1033–1037 (2006).
Zandbelt, M. M. et al. Etanercept in the treatment of patients with primary Sjögren's syndrome: a pilot study. J. Rheumatol. 31, 96–101 (2004).
Devauchelle-Pensec, V. et al. Improvement of Sjögren's syndrome after two infusions of rituximab (anti-CD20). Arthritis Rheum. 57, 310–317 (2007).
Carubbi, F. et al. Efficacy and safety of rituximab treatment in early primary Sjögren's syndrome: a prospective, multi-center, follow-up study. Arthritis Res. Ther. 15, R172 (2013).
Cornec, D. et al. Do high numbers of salivary gland-infiltrating B cells predict better or worse outcomes after rituximab in patients with primary Sjögren's syndrome? Ann. Rheum. Dis. 75, e33 (2016).
Christodoulou, M. I., Kapsogeorgou, E. K. & Moutsopoulos, H. M. Characteristics of the minor salivary gland infiltrates in Sjögren's syndrome. J. Autoimmun. 34, 400–407 (2010).
Thabet, Y. et al. Epigenetic dysregulation in salivary glands from patients with primary Sjögren's syndrome may be ascribed to infiltrating B cells. J. Autoimmun. 41, 175–181 (2013).
Kong, L. et al. Fas and Fas ligand expression in the salivary glands of patients with primary Sjögren's syndrome. Arthritis Rheum. 40, 87–97 (1997).
Varin, M. M. et al. In Sjögren's syndrome, B lymphocytes induce epithelial cells of salivary glands into apoptosis through protein kinase C delta activation. Autoimmun. Rev. 11, 252–258 (2012).
Spachidou, M. P. et al. Expression of functional Toll-like receptors by salivary gland epithelial cells: increased mRNA expression in cells derived from patients with primary Sjögren's syndrome. Clin. Exp. Immunol. 147, 497–503 (2007).
Zheng, L., Zhang, Z., Yu, C. & Yang, C. Expression of Toll-like receptors 7, 8, and 9 in primary Sjögren's syndrome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 109, 844–850 (2010).
Kapsogeorgou, E. K., Moutsopoulos, H. M. & Manoussakis, M. N. Functional expression of a costimulatory B7.2 (CD86) protein on human salivary gland epithelial cells that interacts with the CD28 receptor, but has reduced binding to CTLA4. J. Immunol. 166, 3107–3113 (2001).
Fox, R. I., Bumol, T., Fantozzi, R., Bone, R. & Schreiber, R. Expression of histocompatibility antigen HLA-DR by salivary gland epithelial cells in Sjögren's syndrome. Arthritis Rheum. 29, 1105–1111 (1986).
Kapsogeorgou, E. K., Dimitriou, I. D., Abu-Helu, R. F., Moutsopoulos, H. M. & Manoussakis, M. N. Activation of epithelial and myoepithelial cells in the salivary glands of patients with Sjögren's syndrome: high expression of intercellular adhesion molecule-1 (ICAM.1) in biopsy specimens and cultured cells. Clin. Exp. Immunol. 124, 126–133 (2001).
Yannopoulos, D. I. et al. Conjunctival epithelial cells from patients with Sjögren's syndrome inappropriately express major histocompatibility complex molecules, La(SSB) antigen, and heat-shock proteins. J. Clin. Immunol. 12, 259–265 (1992).
Hamm-Alvarez, S. F. et al. Tear cathepsin S as a candidate biomarker for Sjögren's syndrome. Arthritis Rheumatol. 66, 1872–1881 (2014).
Xanthou, G. et al. 'Lymphoid' chemokine messenger RNA expression by epithelial cells in the chronic inflammatory lesion of the salivary glands of Sjögren's syndrome patients: possible participation in lymphoid structure formation. Arthritis Rheum. 44, 408–418 (2001).
Brkic, Z. & Versnel, M. A. Type I IFN signature in primary Sjögren's syndrome patients. Expert Rev. Clin. Immunol. 10, 457–467 (2014).
Guiducci, C. et al. PI3K is critical for the nuclear translocation of IRF-7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. J. Exp. Med. 205, 315–322 (2008).
Ittah, M. et al. B cell-activating factor of the tumor necrosis factor family (BAFF) is expressed under stimulation by interferon in salivary gland epithelial cells in primary Sjögren's syndrome. Arthritis Res. Ther. 8, R51 (2006).
Mackay, F. et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 190, 1697–1710 (1999).
Mariette, X. et al. The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjögren's syndrome. Ann. Rheum. Dis. 62, 168–171 (2003).
Pers, J. O. et al. BAFF overexpression is associated with autoantibody production in autoimmune diseases. Ann. NY Acad. Sci. 1050, 34–39 (2005).
Gottenberg, J. E. et al. Serum levels of beta2-microglobulin and free light chains of immunoglobulins are associated with systemic disease activity in primary Sjögren's syndrome. Data at enrollment in the prospective ASSESS cohort. PLoS ONE 8, e59868 (2013).
Quartuccio, L. et al. BLyS upregulation in Sjögren's syndrome associated with lymphoproliferative disorders, higher ESSDAI score and B-cell clonal expansion in the salivary glands. Rheumatol. (Oxford) 52, 276–281 (2013).
Drayton, D. L., Ying, X., Lee, J., Lesslauer, W. & Ruddle, N. H. Ectopic LTαβ directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J. Exp. Med. 197, 1153–1163 (2003).
Goodnow, C. C., Vinuesa, C. G., Randall, K. L., Mackay, F. & Brink, R. Control systems and decision making for antibody production. Nat. Immunol. 11, 681–688 (2010).
Vinuesa, C. G. & Cyster, J. G. How T cells earn the follicular rite of passage. Immunity 35, 671–680 (2011).
Ito, T. et al. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity 28, 870–880 (2008).
Nguyen, C. Q., Hu, M. H., Li, Y., Stewart, C. & Peck, A. B. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjögren's syndrome: findings in humans and mice. Arthritis Rheum. 58, 734–743 (2008).
Sakai, A., Sugawara, Y., Kuroishi, T., Sasano, T. & Sugawara, S. Identification of IL-18 and Th17 cells in salivary glands of patients with Sjögren's syndrome, and amplification of IL-17-mediated secretion of inflammatory cytokines from salivary gland cells by IL-18. J. Immunol. 181, 2898–2906 (2008).
Katsifis, G. E., Rekka, S., Moutsopoulos, N. M., Pillemer, S. & Wahl, S. M. Systemic and local interleukin-17 and linked cytokines associated with Sjögren's syndrome immunopathogenesis. Am. J. Pathol. 175, 1167–1177 (2009).
Fleige, H. et al. IL-17-induced CXCL12 recruits B cells and induces follicle formation in BALT in the absence of differentiated FDCs. J. Exp. Med. 211, 643–651 (2014).
Lemoine, S., Morva, A., Youinou, P. & Jamin, C. Regulatory B cells in autoimmune diseases: how do they work? Ann. NY Acad. Sci. 1173, 260–267 (2009).
Lemoine, S., Morva, A., Youinou, P. & Jamin, C. Human T cells induce their own regulation through activation of B cells. J. Autoimmun. 36, 228–238 (2011).
Nouel, A. et al. B-cells induce regulatory T cells through TGF-β/IDO production in A CTLA-4 dependent manner. J. Autoimmun 59, 53–60 (2015).
Rosser, E. C. & Mauri, C. Regulatory B cells: origin, phenotype, and function. Immunity 42, 607–612 (2015).
Blair, P. A. et al. CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 32, 129–140 (2010).
Devauchelle-Pensec, V. et al. Which and how many patients should be included in randomised controlled trials to demonstrate the efficacy of biologics in primary Sjögren's syndrome? PLoS ONE 10, e0133907 (2015).
Jousse-Joulin, S. et al. Brief report: ultrasonographic assessment of salivary gland response to rituximab in primary Sjögren's syndrome. Arthritis Rheumatol. 67, 1623–1628 (2015).
Cornec, D. et al. Development of the Sögren's Syndrome Responder Index, a data-driven composite endpoint for assessing treatment efficacy. Rheumatology (Oxford) 54, 1699–1708 (2015).
Oni, C. et al. Eligibility for clinical trials in primary Sjögren's syndrome: lessons from the UK Primary Sjögren's Syndrome Registry. Rheumatology (Oxford) (2015).
Ono, M. et al. Therapeutic effect of cevimeline on dry eye in patients with Sjögren's syndrome: a randomized, double-blind clinical study. Am. J. Ophthalmol. 138, 6–17 (2004).
Price, E. J., Rigby, S. P., Clancy, U. & Venables, P. J. A double blind placebo controlled trial of azathioprine in the treatment of primary Sjögren's syndrome. J. Rheumatol. 25, 896–899 (1998).
Drosos, A. A., Skopouli, F. N., Costopoulos, J. S., Papadimitriou, C. S. & Moutsopoulos, H. M. Cyclosporin A (CyA) in primary Sjögren's syndrome: a double blind study. Ann. Rheum. Dis. 45, 732–735 (1986).
Norheim, K. B., Harboe, E., Goransson, L. G. & Omdal, R. Interleukin-1 inhibition and fatigue in primary Sjögren's syndrome — a double blind, randomised clinical trial. PLoS ONE 7, e30123 (2012).
Acknowledgements
The authors thank all members of the Brest Diagnosis Primary Sjögren Cohort Study Group: D. Cornec, S. Jousse-Joulin, T. Marhadour, D. Guellec, S. Boisramé-Gastrin, B. Cochener, M. Roguedas-Contios, M. Chastaing, V. Griner-Abraham, F. Couturaud, J. B. Noury, S. Genestet, C. Hanrotel, Y. Renaudineau, P. Marcorelles, S. Costa and Y. Gauvin.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article and contributed to discussions of content, writing the article, and reviewing and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J.-O.P. declares that he has received honoraria from Novartis. A.S. declares that he has received funds or honoraria from Abbvie, Bristol Myers Squibb, Chugai, GlaxoSmithKline, MSD, Novartis, Pfizer, Roche and UCB.. V.D.-P. declares that she has received funds or honoraria from Abbvie, Bristol Myers Squibb, Chugai, MSD, Novartis, Roche and UCB.
Related links
Supplementary information
Supplementary information S1 (table)
Main immunopathological mechanisms involved in primary Sjögren syndrome (pSS) and potential therapeutic targets (PDF 1515 kb)
Glossary
- Focus score
-
A histopathological score based on the number of mononuclear cell infiltrates containing at least 50 inflammatory cells in a 4mm2 glandular section of the minor salivary gland.
- Ocular staining score
-
(OSS). A score that, similarly to the van Bistjerveld score, is based on a test using a dye (rose bengal or lissamine green) to evaluate damage to conjunctival and corneal epithelial cells. Slight differences exist in the interpretation of these scores: a van Bistjerveld score of 4 is equivalent to an OSS of 5.
- Schirmer's test
-
An objective evaluation of eye dryness using calibrated strips of filter paper placed within the eyelid.
- Salivary flow rate
-
The patient passively drools every minute for 15 min into a 50 ml tube, without or after stimulation. The collected samples are weighed using an analytical balance and expressed in ml per min.
- Chronic suppurative sialadenitis
-
Sudden onset of pain, swelling, indurated, tender major salivary gland (most commonly the parotid gland) with purulence from duct due to bacterial infection.
- Sialolithiasis
-
Postprandial salivary pain and swelling caused by the formation of stones (salts and proteins, predominantly calcium carbonate) in the ductal system; the submandibular gland is most often affected.
Rights and permissions
About this article
Cite this article
Saraux, A., Pers, JO. & Devauchelle-Pensec, V. Treatment of primary Sjögren syndrome. Nat Rev Rheumatol 12, 456–471 (2016). https://doi.org/10.1038/nrrheum.2016.100
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2016.100
This article is cited by
-
The composition and function profile of the gut microbiota of patients with primary Sjögren’s syndrome
Clinical Rheumatology (2023)
-
Low-dose interleukin-2 can improve salivary secretion but not lymphocyte infiltration of salivary glands in a murine model of Sjögren’s syndrome
BMC Immunology (2022)
-
Exposure-lag-response associations between extreme environmental conditions and primary Sjögren’s syndrome
Clinical Rheumatology (2022)
-
An unusual cause of immune complex-mediated membranoproliferative glomerulonephritis in a child: Answers
Pediatric Nephrology (2021)
-
Salivary Glands and Periodontal Changes in a Population of Sjögren's and Sicca Syndrome Treated by Pilocarpine: A Pilot Study
Rheumatology and Therapy (2021)