Key Points
-
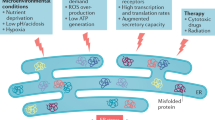
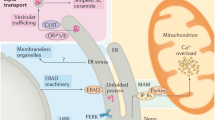
The endoplasmic reticulum (ER) constantly monitors the amount and conformational status of secreted and membrane-related proteins
-
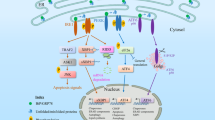
The ER rapidly activates multiple signalling pathways in response to changes in the quality and quantity of the proteins it processes, levels of reactive oxygen species and metabolic changes
-
The ER is an intracellular nexus of the innate and adaptive immune response pathways
-
Disrupted ER homeostasis has an important role in the pathogenesis of many diseases, including several rheumatic diseases
-
ER stress signalling pathways are important in several immune and somatic cell types implicated in disease pathways: fibroblast-like synoviocytes, myeloid cells, myocytes, endothelial cells, B cells, chondrocytes and epithelial cells
-
Future therapies that target ER signalling pathways have potential applications in many rheumatic diseases
Abstract
Rheumatic diseases represent a heterogeneous group of inflammatory conditions, many of which involve chronic activation of both innate and adaptive immune responses by multiple genetic and environmental factors. These immune responses involve the secretion of excessive amounts of cytokines and other signalling mediators by activated immune cells. The endoplasmic reticulum (ER) is the cellular organelle that directs the folding, processing and trafficking of membrane-bound and secreted proteins, including many key components of the immune response. Maintaining homeostasis in the ER is critical to cell function and survival. Consequently, elaborate mechanisms have evolved to sense and respond to ER stress through three main signalling pathways that together comprise the unfolded protein response (UPR). Activation of the UPR can rapidly resolve the accumulation of misfolded proteins, direct permanent changes in the size and function of cells during differentiation, and critically influence the immune response and inflammation. Recognition of the importance of ER stress and UPR signalling pathways in normal and dysregulated immune responses has greatly increased in the past few years. This Review discusses several settings in which ER stress contributes to the pathogenesis of rheumatic diseases and considers some of the therapeutic opportunities that these discoveries provide.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Brandizzi, F. & Barlowe, C. Organization of the ER–Golgi interface for membrane traffic control. Nat. Rev. Mol. Cell Biol. 14, 382–392 (2013).
Kohno, K., Normington, K., Sambrook, J., Gething, M. J. & Mori, K. The promoter region of the yeast KAR2 (BiP) gene contains a regulatory domain that responds to the presence of unfolded proteins in the endoplasmic reticulum. Mol. Cell. Biol. 13, 877–890 (1993).
Cox, J. S., Shamu, C. E. & Walter, P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 73, 1197–1206 (1993).
Shamu, C. E., Cox, J. S. & Walter, P. The unfolded-protein-response pathway in yeast. Trends Cell Biol. 4, 56–60 (1994).
Harding, H. P. et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 (2003).
Janssens, S., Pulendran, B. & Lambrecht, B. N. Emerging functions of the unfolded protein response in immunity. Nat. Immunol. 15, 910–919 (2014).
Bettigole, S. E. & Glimcher, L. H. Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol. 33, 107–138 (2015).
Araki, K. & Nagata, K. Protein folding and quality control in the ER. Cold Spring Harb. Perspect. Biol. 4, a015438 (2012).
Oyadomari, S. et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J. Clin. Invest. 109, 525–532 (2002).
Ron, D. Proteotoxicity in the endoplasmic reticulum: lessons from the Akita diabetic mouse. J. Clin. Invest. 109, 443–445 (2002).
Chen, B., Retzlaff, M., Roos, T. & Frydman, J. Cellular strategies of protein quality control. Cold Spring Harb. Perspect. Biol. 3, a004374 (2011).
Smith, M. H., Ploegh, H. L. & Weissman, J. S. Road to ruin: targeting proteins for degradation in the endoplasmic reticulum. Science 334, 1086–1090 (2011).
Yang, Z. & Klionsky, D. J. Eaten alive: a history of macroautophagy. Nat. Cell Biol. 12, 814–822 (2010).
Stolz, A., Ernst, A. & Dikic, I. Cargo recognition and trafficking in selective autophagy. Nat. Cell Biol. 16, 495–501 (2014).
Kroemer, G., Marino, G. & Levine, B. Autophagy and the integrated stress response. Mol. Cell 40, 280–293 (2010).
Khaminets, A. et al. Regulation of endoplasmic reticulum turnover by selective autophagy. Nature 522, 354–358 (2015).
Gardner, B. M., Pincus, D., Gotthardt, K., Gallagher, C. M. & Walter, P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb. Perspect. Biol. 5, a013169 (2013).
Yoshida, H., Matsui, T., Yamamoto, A., Okada, T. & Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 (2001).
Calfon, M. et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415, 92–96 (2002).
Acosta-Alvear, D. et al. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol. Cell 27, 53–66 (2007).
Lee, A. H., Iwakoshi, N. N. & Glimcher, L. H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 23, 7448–7459 (2003).
Shaffer, A. L. et al. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 21, 81–93 (2004).
Hollien, J. & Weissman, J. S. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313, 104–107 (2006).
Lin, J. H. et al. IRE1 signaling affects cell fate during the unfolded protein response. Science 318, 944–949 (2007).
Han, D. et al. IRE1α kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell 138, 562–575 (2009).
Martino, M. B. et al. The ER stress transducer IRE1β is required for airway epithelial mucin production. Mucosal Immunol. 6, 639–654 (2013).
Tsuru, A. et al. Negative feedback by IRE1β optimizes mucin production in goblet cells. Proc. Natl Acad. Sci. USA 110, 2864–2869 (2013).
Iwawaki, T. et al. Translational control by the ER transmembrane kinase/ribonuclease IRE1 under ER stress. Nat. Cell Biol. 3, 158–164 (2001).
Harding, H. P., Zhang, Y. & Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271–274 (1999).
Harding, H. P., Zhang, Y., Bertolotti, A., Zeng, H. & Ron, D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 5, 897–904 (2000).
Vattem, K. M. & Wek, R. C. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl Acad. Sci. USA 101, 11269–11274 (2004).
Fawcett, T. W., Martindale, J. L., Guyton, K. Z., Hai, T. & Holbrook, N. J. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem. J. 339, 135–141 (1999).
Harding, H. P. et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6, 1099–1108 (2000).
Ma, Y., Brewer, J. W., Diehl, J. A. & Hendershot, L. M. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J. Mol. Biol. 318, 1351–1365 (2002).
Zinszner, H. et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 12, 982–995 (1998).
Marciniak, S. J. et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 18, 3066–3077 (2004).
Cullinan, S. B. et al. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell. Biol. 23, 7198–7209 (2003).
Haze, K., Yoshida, H., Yanagi, H., Yura, T. & Mori, K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 10, 3787–3799 (1999).
Ye, J. et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 6, 1355–1364 (2000).
Shen, J., Chen, X., Hendershot, L. & Prywes, R. ER stress regulation of ATF6 localization by dissociation and unmasking of Golgi localization signals. Dev. Cell 3, 99–111 (2002).
Yoshida, H., Haze, K., Yanagi, H., Yura, T. & Mori, K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins: involvement of basic leucine zipper transcription factors. J. Biol. Chem. 273, 33741–33749 (1998).
Adachi, Y. et al. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struct. Funct. 33, 75–89 (2008).
Wu, J. et al. ATF6α optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev. Cell 13, 351–364 (2007).
Reimold, A. M. et al. An essential role in liver development for transcription factor XBP-1. Genes Dev. 14, 152–157 (2000).
Urano, F. et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287, 664–666 (2000).
Zhang, K. et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell 124, 587–599 (2006).
Murakami, T. et al. Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat. Cell Biol. 11, 1205–1211 (2009).
Saito, A. et al. Regulation of endoplasmic reticulum stress response by a BBF2H7-mediated Sec23a pathway is essential for chondrogenesis. Nat. Cell Biol. 11, 1197–1204 (2009).
Hino, K. et al. Master regulator for chondrogenesis, Sox9, regulates transcriptional activation of the endoplasmic reticulum stress transducer BBF2H7/CREB3L2 in chondrocytes. J. Biol. Chem. 289, 13810–13820 (2014).
Cameron, T. L. et al. Cartilage-specific ablation of XBP1 signaling in mouse results in a chondrodysplasia characterized by reduced chondrocyte proliferation and delayed cartilage maturation and mineralization. Osteoarthritis Cartilage 23, 661–670 (2015).
Harding, H. P. et al. Diabetes mellitus and exocrine pancreatic dysfunction in Perk−/− mice reveals a role for translational control in secretory cell survival. Mol. Cell 7, 1153–1163 (2001).
Zhang, P. et al. The PERK eukaryotic initiation factor 2α kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol. Cell. Biol. 22, 3864–3874 (2002).
Lee, A. H., Chu, G. C., Iwakoshi, N. N. & Glimcher, L. H. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 24, 4368–4380 (2005).
Kaser, A. et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134, 743–756 (2008).
Iwakoshi, N. N. et al. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat. Immunol. 4, 321–329 (2003).
Bettigole, S. E. et al. The transcription factor XBP1 is selectively required for eosinophil differentiation. Nat. Immunol. 16, 829–837 (2015).
Osorio, F. et al. The unfolded-protein-response sensor IRE-1alpha regulates the function of CD8α+ dendritic cells. Nat. Immunol. 15, 248–257 (2014).
Iwakoshi, N. N., Pypaert, M. & Glimcher, L. H. The transcription factor XBP-1 is essential for the development and survival of dendritic cells. J. Exp. Med. 204, 2267–2275 (2007).
Brunsing, R. et al. B- and T-cell development both involve activity of the unfolded protein response pathway. J. Biol. Chem. 283, 17954–17961 (2008).
Dickhout, J. G. et al. Induction of the unfolded protein response after monocyte to macrophage differentiation augments cell survival in early atherosclerotic lesions. FASEB J. 25, 576–589 (2011).
Tohmonda, T. et al. IRE1α/XBP1-mediated branch of the unfolded protein response regulates osteoclastogenesis. J. Clin. Invest. 125, 3269–3279 (2015).
Ozcan, U. et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 (2004).
Keestra-Gounder, A. M. et al. NOD1 and NOD2 signalling links ER stress with inflammation. Nature 532, 394–397 (2016).
Kaneko, M., Niinuma, Y. & Nomura, Y. Activation signal of nuclear factor-kB in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol. Pharm. Bull. 26, 931–935 (2003).
Hu, P., Han, Z., Couvillon, A. D., Kaufman, R. J. & Exton, J. H. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1α-mediated NF-κB activation and down-regulation of TRAF2 expression. Mol. Cell. Biol. 26, 3071–3084 (2006).
Jiang, H. Y. et al. Phosphorylation of the α subunit of eukaryotic initiation factor 2 is required for activation of NF-κB in response to diverse cellular stresses. Mol. Cell. Biol. 23, 5651–5663 (2003).
Cho, J. A. et al. The unfolded protein response element IRE1α senses bacterial proteins invading the ER to activate RIG-I and innate immune signaling. Cell Host Microbe 13, 558–569 (2013).
Petrasek, J. et al. STING–IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc. Natl Acad. Sci. USA 110, 16544–16549 (2013).
Hattori, T., Ohoka, N., Hayashi, H. & Onozaki, K. C/EBP homologous protein (CHOP) up-regulates IL-6 transcription by trapping negative-regulating NF-IL6 isoform. FEBS Lett. 541, 33–39 (2003).
Marjon, P. L., Bobrovnikova-Marjon, E. V. & Abcouwer, S. F. Expression of the pro-angiogenic factors vascular endothelial growth factor and interleukin-8/CXCL8 by human breast carcinomas is responsive to nutrient deprivation and endoplasmic reticulum stress. Mol. Cancer 3, 4 (2004).
Gora, S. et al. Phospholipolyzed LDL induces an inflammatory response in endothelial cells through endoplasmic reticulum stress signaling. FASEB J. 24, 3284–3297 (2010).
Maguire, J. A., Mulugeta, S. & Beers, M. F. Endoplasmic reticulum stress induced by surfactant protein C BRICHOS mutants promotes proinflammatory signaling by epithelial cells. Am. J. Respir. Cell Mol. Biol. 44, 404–414 (2011).
Smith, J. A. et al. Endoplasmic reticulum stress and the unfolded protein response are linked to synergistic IFN-β induction via X-box binding protein 1. Eur. J. Immunol. 38, 1194–1203 (2008).
Hu, F. et al. ER stress and its regulator X-box-binding protein-1 enhance polyIC-induced innate immune response in dendritic cells. Eur. J. Immunol. 41, 1086–1097 (2011).
DeLay, M. L. et al. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 60, 2633–2643 (2009).
Colbert, R. A., DeLay, M. L., Klenk, E. I. & Layh-Schmitt, G. From HLA-B27 to spondyloarthritis: a journey through the ER. Immunol. Rev. 233, 181–202 (2010).
Clavarino, G. et al. Protein phosphatase 1 subunit Ppp1r15a/GADD34 regulates cytokine production in polyinosinic:polycytidylic acid-stimulated dendritic cells. Proc. Natl Acad. Sci. USA 109, 3006–3011 (2012).
Goodall, J. C. et al. Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc. Natl Acad. Sci. USA 107, 17698–17703 (2010).
Shenderov, K. et al. Cutting edge: endoplasmic reticulum stress licenses macrophages to produce mature IL-1β in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway. J. Immunol. 192, 2029–2033 (2014).
Menu, P. et al. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death Dis. 3, e261 (2012).
Lerner, A. G. et al. IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 16, 250–264 (2012).
Oslowski, C. M. et al. Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metab. 16, 265–273 (2012).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
Anthony, T. G. & Wek, R. C. TXNIP switches tracks toward a terminal UPR. Cell Metab. 16, 135–137 (2012).
Edwan, J. H., Goldbach-Mansky, R. & Colbert, R. A. Identification of interleukin-1β-producing monocytes that are susceptible to pyronecrotic cell death in patients with neonatal-onset multisystem inflammatory disease. Arthritis Rheumatol. 67, 3286–3297 (2015).
Thacker, J. D. et al. 1-Peptidyl-2-arachidonoyl-3-stearoyl-sn-glyceride: an immunologically active lipopeptide from goat serum (Capra hircus) is an endogenous damage-associated molecular pattern. J. Nat. Prod. 72, 1993–1999 (2009).
Overley-Adamson, B. et al. Targeting the unfolded protein response, XBP1, and the NLRP3 inflammasome in fibrosis and cancer. Cancer Biol. Ther. 15, 452–462 (2014).
Broderick, L., De Nardo, D., Franklin, B. S., Hoffman, H. M. & Latz, E. The inflammasomes and autoinflammatory syndromes. Annu. Rev. Pathol. 10, 395–424 (2015).
Bartoszewski, R. et al. The unfolded protein response (UPR)-activated transcription factor X-box-binding protein 1 (XBP1) induces microRNA-346 expression that targets the human antigen peptide transporter 1 (TAP1) mRNA and governs immune regulatory genes. J. Biol. Chem. 286, 41862–41870 (2011).
Layh-Schmitt, G., Yang, E. Y., Kwon, G. & Colbert, R. A. HLA-B27 alters the response to tumor necrosis factor α and promotes osteoclastogenesis in bone marrow monocytes from HLA-B27-transgenic rats. Arthritis Rheum. 65, 2123–2131 (2013).
Suwara, M. I. et al. IL-1α released from damaged epithelial cells is sufficient and essential to trigger inflammatory responses in human lung fibroblasts. Mucosal Immunol. 7, 684–693 (2014).
Martinon, F., Chen, X., Lee, A. H. & Glimcher, L. H. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 11, 411–418 (2010).
Woo, C. W., Kutzler, L., Kimball, S. R. & Tabas, I. Toll-like receptor activation suppresses ER stress factor CHOP and translation inhibition through activation of eIF2B. Nat. Cell Biol. 14, 192–200 (2012).
Perri, E. R., Thomas, C. J., Parakh, S., Spencer, D. M. & Atkin, J. D. The unfolded protein response and the role of protein disulfide isomerase in neurodegeneration. Front. Cell Dev. Biol. 3, 80 (2015).
Osorio, F., Lambrecht, B. & Janssens, S. The UPR and lung disease. Semin. Immunopathol. 35, 293–306 (2013).
Zhou, A. X. & Tabas, I. The UPR in atherosclerosis. Semin. Immunopathol. 35, 321–332 (2013).
Manie, S. N., Lebeau, J. & Chevet, E. Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. 3. Orchestrating the unfolded protein response in oncogenesis: an update. Am. J. Physiol. Cell Physiol. 307, C901–C907 (2014).
Iwawaki, T. & Oikawa, D. The role of the unfolded protein response in diabetes mellitus. Semin. Immunopathol. 35, 333–350 (2013).
Smith, J. A. A new paradigm: innate immune sensing of viruses via the unfolded protein response. Front. Microbiol. 5, 222 (2014).
Adolph, T. E., Niederreiter, L., Blumberg, R. S. & Kaser, A. Endoplasmic reticulum stress and inflammation. Dig. Dis. 30, 341–346 (2012).
Adolph, T. E. et al. Paneth cells as a site of origin for intestinal inflammation. Nature 503, 272–276 (2013).
McInnes, I. B. & Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365, 2205–2219 (2011).
Wegner, N. et al. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 62, 2662–2672 (2010).
Lugli, E. B. et al. Expression of citrulline and homocitrulline residues in the lungs of non-smokers and smokers: implications for autoimmunity in rheumatoid arthritis. Arthritis Res. Ther. 17, 9 (2015).
Klareskog, L. & Catrina, A. I. Autoimmunity: lungs and citrullination. Nat. Rev. Rheumatol. 11, 261–262 (2015).
Qu, Z. et al. Local proliferation of fibroblast-like synoviocytes contributes to synovial hyperplasia. Results of proliferating cell nuclear antigen/cyclin, c-myc, and nucleolar organizer region staining. Arthritis Rheum. 37, 212–220 (1994).
Pap, T., Muller-Ladner, U., Gay, R. E. & Gay, S. Fibroblast biology: role of synovial fibroblasts in the pathogenesis of rheumatoid arthritis. Arthritis Res. 2, 361–367 (2000).
Buckley, C. D. et al. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 22, 199–204 (2001).
Park, Y. J., Yoo, S. A. & Kim, W. U. Role of endoplasmic reticulum stress in rheumatoid arthritis pathogenesis. J. Korean Med. Sci. 29, 2–11 (2014).
Gao, B., Calhoun, K. & Fang, D. The proinflammatory cytokines IL-1β and TNF-α induce the expression of Synoviolin, an E3 ubiquitin ligase, in mouse synovial fibroblasts via the Erk1/2–ETS1 pathway. Arthritis Res. Ther. 8, R172 (2006).
Amano, T. et al. Synoviolin/Hrd1, an E3 ubiquitin ligase, as a novel pathogenic factor for arthropathy. Genes Dev. 17, 2436–2449 (2003).
Yamasaki, S., Yagishita, N., Tsuchimochi, K., Nishioka, K. & Nakajima, T. Rheumatoid arthritis as a hyper-endoplasmic-reticulum-associated degradation disease. Arthritis Res. Ther. 7, 181–186 (2005).
Klaasen, R. et al. Synovial synoviolin in relation to response to TNF blockade in patients with rheumatoid arthritis and psoriatic arthritis. Ann. Rheum. Dis. 71, 1260–1261 (2012).
Yoo, S. A. et al. A novel pathogenic role of the ER chaperone GRP78/BiP in rheumatoid arthritis. J. Exp. Med. 209, 871–886 (2012).
Corrigall, V. M. et al. The human endoplasmic reticulum molecular chaperone BiP is an autoantigen for rheumatoid arthritis and prevents the induction of experimental arthritis. J. Immunol. 166, 1492–1498 (2001).
Blass, S. et al. The stress protein BiP is overexpressed and is a major B and T cell target in rheumatoid arthritis. Arthritis Rheum. 44, 761–771 (2001).
Brownlie, R. J. et al. Treatment of murine collagen-induced arthritis by the stress protein BiP via interleukin-4-producing regulatory T cells: a novel function for an ancient protein. Arthritis Rheum. 54, 854–863 (2006).
Shoda, H. et al. Autoantigen BiP-derived HLA-DR4 epitopes differentially recognized by effector and regulatory T cells in rheumatoid arthritis. Arthritis Rheumatol. 67, 1171–1181 (2015).
Taurog, J. D., Chhabra, A. & Colbert, R. A. Axial spondyloarthritis and ankylosing spondylitis. N. Engl. J. Med. 374, 2563–2574 (2016).
Wright, C. et al. Detection of multiple autoantibodies in patients with ankylosing spondylitis using nucleic acid programmable protein arrays. Mol. Cell. Proteomics 11, M9.00384 (2012).
Baraliakos, X., Baerlecken, N., Witte, T., Heldmann, F. & Braun, J. High prevalence of anti-CD74 antibodies specific for the HLA class II-associated invariant chain peptide (CLIP) in patients with axial spondyloarthritis. Ann. Rheum. Dis. 73, 1079–1082 (2014).
Tsui, F. W., Tsui, H. W., Las Heras, F., Pritzker, K. P. & Inman, R. D. Serum levels of novel noggin and sclerostin-immune complexes are elevated in ankylosing spondylitis. Ann. Rheum. Dis. 73, 1873–1879 (2014).
Kim, Y. G. et al. Role of protein phosphatase magnesium-dependent 1A and anti-protein phosphatase magnesium-dependent 1A autoantibodies in ankylosing spondylitis. Arthritis Rheumatol. 66, 2793–2803 (2014).
Van den Bosch, F. & Deodhar, A. Treatment of spondyloarthritis beyond TNF-alpha blockade. Best Pract. Res. Clin. Rheumatol. 28, 819–827 (2014).
Brown, M. A., Kenna, T. & Wordsworth, B. P. Genetics of ankylosing spondylitis — insights into pathogenesis. Nat. Rev. Rheumatol. 12, 81–91 (2016).
Smith, J. A. & Colbert, R. A. Review: the interleukin-23/interleukin-17 axis in spondyloarthritis pathogenesis: Th17 and beyond. Arthritis Rheumatol. 66, 231–241 (2014).
May, E., Satumitra, N., Dorris, M. I. & Taurog, J. D. No evidence for clonal T cell expansion in early stage arthritis of HLA-B27 transgenic rats. Arthritis Rheum. 43, S263 (2000).
Glatigny, S. et al. Proinflammatory Th17 cells are expanded and induced by dendritic cells in spondylarthritis-prone HLA-B27-transgenic rats. Arthritis Rheum. 64, 110–120 (2012).
Sherlock, J. P. et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4−CD8− entheseal resident T cells. Nat. Med. 18, 1069–1076 (2012).
Colbert, R. A., Tran, T. M. & Layh-Schmitt, G. HLA-B27 misfolding and ankylosing spondylitis. Mol. Immunol. 57, 44–51 (2014).
Bowness, P. HLA-B27. Annu. Rev. Immunol. 33, 29–48 (2015).
Bowness, P. et al. Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J. Immunol. 186, 2672–2680 (2011).
Turner, M. J., Delay, M. L., Bai, S., Klenk, E. & Colbert, R. A. HLA-B27 up-regulation causes accumulation of misfolded heavy chains and correlates with the magnitude of the unfolded protein response in transgenic rats: implications for the pathogenesis of spondylarthritis-like disease. Arthritis Rheum. 56, 215–223 (2007).
Turner, M. J. et al. HLA-B27 misfolding in transgenic rats is associated with activation of the unfolded protein response. J. Immunol. 175, 2438–2448 (2005).
Mear, J. P. et al. Misfolding of HLA-B27 as a result of its B pocket suggests a novel mechanism for its role in susceptibility to spondyloarthropathies. J. Immunol. 163, 6665–6670 (1999).
Feng, Y., Ding, J., Fan, C. M. & Zhu, P. Interferon-γ contributes to HLA-B27-associated unfolded protein response in spondyloarthropathies. J. Rheumatol. 39, 574–582 (2012).
Smith, J. A. et al. Gene expression analysis of macrophages derived from ankylosing spondylitis patients reveals interferon-γ dysregulation. Arthritis Rheum. 58, 1640–1649 (2008).
Zeng, L., Lindstrom, M. J. & Smith, J. A. Ankylosing spondylitis macrophage production of higher levels of interleukin-23 in response to lipopolysaccharide without induction of a significant unfolded protein response. Arthritis Rheum. 63, 3807–3817 (2011).
Neerinckx, B., Carter, S. & Lories, R. J. No evidence for a critical role of the unfolded protein response in synovium and blood of patients with ankylosing spondylitis. Ann. Rheum. Dis. 73, 629–630 (2014).
Dong, W. et al. Upregulation of 78-kDa glucose-regulated protein in macrophages in peripheral joints of active ankylosing spondylitis. Scand. J. Rheumatol. 37, 427–434 (2008).
Dangoria, N. S. et al. HLA-B27 misfolding is associated with aberrant intermolecular disulfide bond formation (dimerization) in the endoplasmic reticulum. J. Biol. Chem. 277, 23459–23468 (2002).
Antoniou, A. N., Ford, S., Taurog, J. D., Butcher, G. W. & Powis, S. J. Formation of HLA-B27 homodimers and their relationship to assembly kinetics. J. Biol. Chem. 279, 8895–8902 (2004).
Jeanty, C. et al. HLA-B27 subtype oligomerization and intracellular accumulation patterns correlate with predisposition to spondyloarthritis. Arthritis Rheumatol. 66, 2113–2123 (2014).
Loll, B. et al. Increased conformational flexibility characterizes HLA-B*27 subtypes associated with ankylosing spondylitis. Arthritis Rheumatol. 68, 1172–1182 (2016).
Bird, L. A. et al. Lymphoblastoid cells express HLA-B27 homodimers both intracellularly and at the cell surface following endosomal recycling. Eur. J. Immunol. 33, 748–759 (2003).
McHugh, K. et al. Expression of aberrant HLA-B27 molecules is dependent on B27 dosage and peptide supply. Ann. Rheum. Dis. 73, 763–770 (2014).
Wong-Baeza, I. et al. KIR3DL2 binds to HLA-B27 dimers and free H chains more strongly than other HLA class I and promotes the expansion of T cells in ankylosing spondylitis. J. Immunol. 190, 3216–3224 (2013).
Ridley, A. et al. Activation-induced killer cell immunoglobulin-like receptor 3DL2 binding to HLA-B27 licenses pathogenic T cell differentiation in spondyloarthritis. Arthritis Rheumatol. 68, 901–914 (2016).
Dalakas, M. C. & Hohlfeld, R. Polymyositis and dermatomyositis. Lancet 362, 971–982 (2003).
Miller, F. W. et al. Genome-wide association study identifies HLA 8.1 ancestral haplotype alleles as major genetic risk factors for myositis phenotypes. Genes Immun. 16, 470–480 (2015).
Miller, F. W. et al. Genome-wide association study of dermatomyositis reveals genetic overlap with other autoimmune disorders. Arthritis Rheum. 65, 3239–3247 (2013).
Rayavarapu, S., Coley, W. & Nagaraju, K. An update on pathogenic mechanisms of inflammatory myopathies. Curr. Opin. Rheumatol. 23, 579–584 (2011).
Fasth, A. E. et al. T cell infiltrates in the muscles of patients with dermatomyositis and polymyositis are dominated by CD28null T cells. J. Immunol. 183, 4792–4799 (2009).
Pandya, J. M. et al. Expanded T cell receptor Vβ-restricted T cells from patients with sporadic inclusion body myositis are proinflammatory and cytotoxic CD28null T cells. Arthritis Rheum. 62, 3457–3466 (2010).
Szodoray, P. et al. Idiopathic inflammatory myopathies, signified by distinctive peripheral cytokines, chemokines and the TNF family members B-cell activating factor and a proliferation inducing ligand. Rheumatology 49, 1867–1877 (2010).
Krystufková, O. et al. Increased serum levels of B cell activating factor (BAFF) in subsets of patients with idiopathic inflammatory myopathies. Ann. Rheum. Dis. 68, 836–843 (2009).
Bilgic, H. et al. Interleukin-6 and type I interferon-regulated genes and chemokines mark disease activity in dermatomyositis. Arthritis Rheum. 60, 3436–3446 (2009).
Gono, T. et al. Interleukin-18 is a key mediator in dermatomyositis: potential contribution to development of interstitial lung disease. Rheumatology (Oxford) 49, 1878–1881 (2010).
Grundtman, C. et al. Effects of HMGB1 on in vitro responses of isolated muscle fibers and functional aspects in skeletal muscles of idiopathic inflammatory myopathies. FASEB J. 24, 570–578 (2010).
Baechler, E. C. et al. An interferon signature in the peripheral blood of dermatomyositis patients is associated with disease activity. Mol. Med. 13, 59–68 (2007).
Walsh, R. J. et al. Type I interferon-inducible gene expression in blood is present and reflects disease activity in dermatomyositis and polymyositis. Arthritis Rheum. 56, 3784–3792 (2007).
Baechler, E. C., Bilgic, H. & Reed, A. M. Type I interferon pathway in adult and juvenile dermatomyositis. Arthritis Res. Ther. 13, 249 (2011).
Liao, A. P. et al. Interferon β is associated with type 1 interferon-inducible gene expression in dermatomyositis. Ann. Rheum. Dis. 70, 831–836 (2011).
Kim, H. J. et al. Endoplasmic reticulum stress markers and ubiquitin-proteasome pathway activity in response to a 200-km run. Med. Sci. Sports Exerc. 43, 18–25 (2011).
Deldicque, L. et al. The unfolded protein response is activated in skeletal muscle by high-fat feeding: potential role in the downregulation of protein synthesis. Am. J. Physiol. Endocrinol. Metab. 299, E695–E705 (2010).
Rayavarapu, S., Coley, W. & Nagaraju, K. Endoplasmic reticulum stress in skeletal muscle homeostasis and disease. Curr. Rheumatol. Rep. 14, 238–243 (2012).
Nagaraju, K. et al. A variety of cytokines and immunologically relevant surface molecules are expressed by normal human skeletal muscle cells under proinflammatory stimuli. Clin. Exp. Immunol. 113, 407–414 (1998).
Emslie-Smith, A. M., Arahata, K. & Engel, A. G. Major histocompatibility complex class I antigen expression, immunolocalization of interferon subtypes, and T cell-mediated cytotoxicity in myopathies. Hum. Pathol. 20, 224–231 (1989).
Nagaraju, K. et al. Conditional up-regulation of MHC class I in skeletal muscle leads to self-sustaining autoimmune myositis and myositis-specific autoantibodies. Proc. Natl Acad. Sci. USA 97, 9209–9214 (2000).
Nagaraju, K. et al. Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis Rheum. 52, 1824–1835 (2005).
Alger, H. M. et al. The role of TRAIL in mediating autophagy in myositis skeletal muscle: a potential nonimmune mechanism of muscle damage. Arthritis Rheum. 63, 3448–3457 (2011).
van den Hoogen, F. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 65, 2737–2747 (2013).
Pope, J. E. & Johnson, S. R. New classification criteria for systemic sclerosis (scleroderma). Rheum. Dis. Clin. North Am. 41, 383–398 (2015).
Gabrielli, A., Avvedimento, E. V. & Krieg, T. Scleroderma. N. Engl. J. Med. 360, 1989–2003 (2009).
Sgonc, R. et al. Endothelial cell apoptosis is a primary pathogenetic event underlying skin lesions in avian and human scleroderma. J. Clin. Invest. 98, 785–792 (1996).
Helmbold, P., Fiedler, E., Fischer, M. & Marsch, W. C. Hyperplasia of dermal microvascular pericytes in scleroderma. J. Cutan. Pathol. 31, 431–440 (2004).
Fleming, J. N. et al. Capillary regeneration in scleroderma: stem cell therapy reverses phenotype? PLoS ONE 3, e1452 (2008).
Korman, B. D. & Criswell, L. A. Recent advances in the genetics of systemic sclerosis: toward biological and clinical significance. Curr. Rheumatol. Rep. 17, 21 (2015).
Lenna, S., Han, R. & Trojanowska, M. Endoplasmic reticulum stress and endothelial dysfunction. IUBMB Life 66, 530–537 (2014).
Arnett, F. C. et al. Increased prevalence of systemic sclerosis in a Native American tribe in Oklahoma. Association with an Amerindian HLA haplotype. Arthritis Rheum. 39, 1362–1370 (1996).
Grigolo, B. et al. Anti-topoisomerase II α autoantibodies in systemic sclerosis — association with pulmonary hypertension and HLA-B35. Clin. Exp. Immunol. 121, 539–543 (2000).
Lenna, S. et al. HLA-B35 upregulates endothelin-1 and downregulates endothelial nitric oxide synthase via endoplasmic reticulum stress response in endothelial cells. J. Immunol. 184, 4654–4661 (2010).
Lenna, S. et al. Increased expression of endoplasmic reticulum stress and unfolded protein response genes in peripheral blood mononuclear cells from patients with limited cutaneous systemic sclerosis and pulmonary arterial hypertension. Arthritis Rheum. 65, 1357–1366 (2013).
Iwawaki, T., Akai, R., Yamanaka, S. & Kohno, K. Function of IRE1 alpha in the placenta is essential for placental development and embryonic viability. Proc. Natl Acad. Sci. USA 106, 16657–16662 (2009).
Zeng, L. et al. Vascular endothelial cell growth-activated XBP1 splicing in endothelial cells is crucial for angiogenesis. Circulation 127, 1712–1722 (2013).
Binet, F. & Sapieha, P. ER Stress and angiogenesis. Cell Metab. 22, 560–575 (2015).
Paridaens, A. et al. Endoplasmic reticulum stress and angiogenesis: is there an interaction between them? Liver Int. 34, e10–e18 (2014).
Ghosh, R. et al. Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PLoS ONE 5, e9575 (2010).
Lenna, S. et al. HLA-B35 and dsRNA induce endothelin-1 via activation of ATF4 in human microvascular endothelial cells. PLoS ONE 8, e56123 (2013).
Ho, Y. Y., Lagares, D., Tager, A. M. & Kapoor, M. Fibrosis — a lethal component of systemic sclerosis. Nat. Rev. Rheumatol. 10, 390–402 (2014).
Eckes, B., Mauch, C., Hüppe, G. & Krieg, T. Differential regulation of transcription and transcript stability of pro-α 1(I) collagen and fibronectin in activated fibroblasts derived from patients with systemic scleroderma. Biochem. J. 315, 549–554 (1996).
Rajkumar, V. S. et al. Shared expression of phenotypic markers in systemic sclerosis indicates a convergence of pericytes and fibroblasts to a myofibroblast lineage in fibrosis. Arthritis Res. Ther. 7, R1113–R1123 (2005).
Baek, H. A. et al. Involvement of endoplasmic reticulum stress in myofibroblastic differentiation of lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 46, 731–739 (2012).
Mohan, C. & Putterman, C. Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis. Nat. Rev. Nephrol. 11, 329–341 (2015).
Tsokos, G. C. Systemic lupus erythematosus. N. Engl. J. Med. 365, 2110–2121 (2011).
Olsen, N. J. & Karp, D. R. Autoantibodies and SLE: the threshold for disease. Nat. Rev. Rheumatol. 10, 181–186 (2014).
Todd, D. J. et al. XBP1 governs late events in plasma cell differentiation and is not required for antigen-specific memory B cell development. J. Exp. Med. 206, 2151–2159 (2009).
Lee, A. H., Iwakoshi, N. N., Anderson, K. C. & Glimcher, L. H. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc. Natl Acad. Sci. USA 100, 9946–9951 (2003).
Obeng, E. A. et al. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood 107, 4907–4916 (2006).
Neubert, K. et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat. Med. 14, 748–755 (2008).
Ichikawa, H. T. et al. Beneficial effect of novel proteasome inhibitors in murine lupus via dual inhibition of type I interferon and autoantibody-secreting cells. Arthritis Rheum. 64, 493–503 (2012).
Muchamuel, T. et al. A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nat. Med. 15, 781–787 (2009).
Garaud, J. C. et al. B cell signature during inactive systemic lupus is heterogeneous: toward a biological dissection of lupus. PLoS ONE 6, e23900 (2011).
Lee, W. S. et al. A pathogenic role for ER stress-induced autophagy and ER chaperone GRP78/BiP in T lymphocyte systemic lupus erythematosus. J. Leukoc. Biol. 97, 425–433 (2015).
Wang, J. et al. Deficiency of IRE1 and PERK signal pathways in systemic lupus erythematosus. Am. J. Med. Sci. 348, 465–473 (2014).
Guo, G. et al. Induction of apoptosis coupled to endoplasmic reticulum stress through regulation of CHOP and JNK in bone marrow mesenchymal stem cells from patients with systemic lupus erythematosus. J. Immunol. Res. 2015, 183738 (2015).
Hirabayashi, Y. et al. The endoplasmic reticulum stress-inducible protein, Herp, is a potential triggering antigen for anti-DNA response. J. Immunol. 184, 3276–3283 (2010).
Hunter, D. J. & Felson, D. T. Osteoarthritis. BMJ 332, 639–642 (2006).
Lane, N. E. Clinical practice: osteoarthritis of the hip. N. Engl. J. Med. 357, 1413–1421 (2007).
Lories, R. J. & Luyten, F. P. The bone–cartilage unit in osteoarthritis. Nat. Rev. Rheumatol. 7, 43–49 (2011).
Luyten, F. P., Lories, R. J., Verschueren, P., se Vlam, K. & Westhovens, R. Contemporary concepts of inflammation, damage and repair in rheumatic diseases. Best Pract. Res. Clin. Rheumatol. 20, 829–848 (2006).
Goldring, M. B. & Goldring, S. R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. NY Acad. Sci. 1192, 230–237 (2010).
Zemmyo, M., Meharra, E. J., Kühn, K., Creighton-Achermann, L. & Lotz, M. Accelerated, aging-dependent development of osteoarthritis in α1 integrin-deficient mice. Arthritis Rheum. 48, 2873–2880 (2003).
Mistry, D., Oue, Y., Chambers, M. G., Kayser, M. V. & Mason, R. M. Chondrocyte death during murine osteoarthritis. Osteoarthritis Cartilage 12, 131–141 (2004).
Kim, H. A., Lee, Y. J., Seong, S. C., Choe, K. W. & Song, Y. W. Apoptotic chondrocyte death in human osteoarthritis. J. Rheumatol. 27, 455–462 (2000).
Thomas, C. M., Fuller, C. J., Whittles, C. E. & Sharif, M. Chondrocyte death by apoptosis is associated with cartilage matrix degradation. Osteoarthritis Cartilage 15, 27–34 (2007).
Walsh, D. A. et al. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology (Oxford) 49, 1852–1861 (2010).
Prasadam, I. et al. ERK-1/2 and p38 in the regulation of hypertrophic changes of normal articular cartilage chondrocytes induced by osteoarthritic subchondral osteoblasts. Arthritis Rheum. 62, 1349–1360 (2010).
Prasadam, I. et al. Osteoarthritic cartilage chondrocytes alter subchondral bone osteoblast differentiation via MAPK signalling pathway involving ERK1/2. Bone 46, 226–235 (2010).
Takada, K. et al. Enhanced apoptotic and reduced protective response in chondrocytes following endoplasmic reticulum stress in osteoarthritic cartilage. Int. J. Exp. Pathol. 92, 232–242 (2011).
Guo, F. J. et al. ATF6 upregulates XBP1S and inhibits ER stress-mediated apoptosis in osteoarthritis cartilage. Cell. Signal. 26, 332–342 (2014).
Uehara, Y. et al. Endoplasmic reticulum stress-induced apoptosis contributes to articular cartilage degeneration via C/EBP homologous protein. Osteoarthritis Cartilage 22, 1007–1017 (2014).
Liu, C., Cao, Y., Yang, X., Shan, P. & Liu, H. Tauroursodeoxycholic acid suppresses endoplasmic reticulum stress in the chondrocytes of patients with osteoarthritis. Int. J. Mol. Med. 36, 1081–1087 (2015).
Lippiello, L., Walsh, T. & Fienhold, M. The association of lipid abnormalities with tissue pathology in human osteoarthritic articular cartilage. Metabolism 40, 571–576 (1991).
Haywood, J. & Yammani, R. R. Free fatty acid palmitate activates unfolded protein response pathway and promotes apoptosis in meniscus cells. Osteoarthritis Cartilage 24, 942–945 (2016).
Nakano, S. et al. Remogliflozin etabonate improves fatty liver disease in diet-induced obese male mice. J. Clin. Exp. Hepatol. 5, 190–198 (2015).
Watkin, L. B. et al. COPA mutations impair ER–Golgi transport and cause hereditary autoimmune-mediated lung disease and arthritis. Nat. Genet. 47, 654–660 (2015).
Todd, D. J., Lee, A. H. & Glimcher, L. H. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat. Rev. Immunol. 8, 663–674 (2008).
Park, H., Bourla, A. B., Kastner, D. L., Colbert, R. A. & Siegel, R. M. Lighting the fires within: the cell biology of autoinflammatory diseases. Nat. Rev. Immunol. 12, 570–580 (2012).
McDermott, M. F. et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell 97, 133–144 (1999).
Lobito, A. A. et al. Abnormal disulfide-linked oligomerization results in ER retention and altered signaling by TNFR1 mutants in TNFR1-associated periodic fever syndrome (TRAPS). Blood 108, 1320–1327 (2006).
Rebelo, S. L. et al. Modeling of tumor necrosis factor receptor superfamily 1A mutants associated with tumor necrosis factor receptor-associated periodic syndrome indicates misfolding consistent with abnormal function. Arthritis Rheum. 54, 2674–2687 (2006).
Todd, I. et al. Mutant tumor necrosis factor receptor associated with tumor necrosis factor receptor-associated periodic syndrome is altered antigenically and is retained within patients' leukocytes. Arthritis Rheum. 56, 2765–2773 (2007).
Simon, A. et al. Concerted action of wild-type and mutant TNF receptors enhances inflammation in TNF receptor 1-associated periodic fever syndrome. Proc. Natl Acad. Sci. USA 107, 9801–9806 (2010).
Dickie, L. J. et al. Involvement of X-box binding protein 1 and reactive oxygen species pathways in the pathogenesis of tumour necrosis factor receptor-associated periodic syndrome. Ann. Rheum. Dis. 71, 2035–2043 (2012).
Liu, Y. et al. Preventing oxidative stress: a new role for XBP1. Cell Death Differ. 16, 847–857 (2009).
Bulua, A. C. et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 208, 519–533 (2011).
Acknowledgements
The research of R.A.C. and F.N. is supported by the NIH and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) Intramural Research Program grant ZO1-AR-041184.
Author information
Authors and Affiliations
Contributions
Both authors researched the data for this article, provided substantial contributions to discussions of its content, wrote the article and undertook review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
R.A.C. declares that he has been the Principal Investigator on a cooperative research and development agreement between Eli Lilly and the US National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). F.N. declares no competing interests.
Rights and permissions
About this article
Cite this article
Navid, F., Colbert, R. Causes and consequences of endoplasmic reticulum stress in rheumatic disease. Nat Rev Rheumatol 13, 25–40 (2017). https://doi.org/10.1038/nrrheum.2016.192
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2016.192
This article is cited by
-
Oxidative stress in Hashimoto’s thyroiditis: possible adjuvant therapies to attenuate deleterious effects
Molecular and Cellular Biochemistry (2023)
-
Insights into the structure and function of the RNA ligase RtcB
Cellular and Molecular Life Sciences (2023)
-
The lupus susceptibility allele DRB1*03:01 encodes a disease-driving epitope
Communications Biology (2022)
-
The VEXAS syndrome from rheumatology perspective: genomic DNA sequencing as available blueprint for diagnosing rheumatic diseases with overlapping haematological or dermatological findings
Clinical Rheumatology (2022)
-
HLA-DRB1 allelic epitopes that associate with autoimmune disease risk or protection activate reciprocal macrophage polarization
Scientific Reports (2021)