Key Points
-
The aetiology and pathophysiology of pelvic-floor dysfunction (PFD) is incompletely understood, although pregnancy, and vaginal birth in particular, cause damage that seems to be insufficiently repaired in some women
-
After nerve and muscle trauma, endogenous repair processes are initiated, but these mechanisms often fail to completely repair the original damage
-
Administration of exogenous drugs, growth factors, cells or even tissue might improve the function of innate repair mechanisms
-
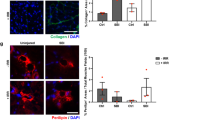
In small-animal models of PFD, the concept of administration of mesenchymal stem cells or factors secreted by these cells has positive effects on pelvic-floor function
-
Clinical trials in patients with either urinary incontinence or faecal incontinence have provided both subjective improvements and objective documentation of improved pelvic floor function after autologous mesenchymal stem cell administration
-
Intuitively, better outcomes seem likely when stem cells are given around the trauma period, before trauma-induced damage becomes a clinical reality; preclinical data are required to support this hypothesis
Abstract
With advancing population age, pelvic-floor dysfunction (PFD) will affect an increasing number of women. Many of these women wish to maintain active lifestyles, indicating an urgent need for effective strategies to treat or, preferably, prevent the occurrence of PFD. Childbirth and pregnancy have both long been recognized as crucial contributing factors in the pathophysiology of PFD. Vaginal delivery of a child is a serious traumatic event, causing anatomical and functional changes in the pelvic floor. Similar changes to those experienced during childbirth can be found in symptomatic women, often many years after delivery. Thus, women with such PFD symptoms might have incompletely recovered from the trauma caused by vaginal delivery. This hypothesis creates the possibility that preventive measures can be initiated around the time of delivery. Secondary prevention has been shown to be beneficial in patients with many other chronic conditions. The current general consensus is that clinicians should aim to minimize the extent of damage during delivery, and aim to optimize healing processes after delivery, therefore preventing later dysfunction. A substantial amount of research investigating the potential of stem-cell injections as a therapeutic strategy for achieving this purpose is currently ongoing. Data from small animal models have demonstrated positive effects of mesenchymal stem-cell injections on the healing process following simulated vaginal birth injury.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
DeLancey, J. O. The hidden epidemic of pelvic floor dysfunction: achievable goals for improved prevention and treatment. Am. J. Obstet. Gynecol. 192, 1488–1495 (2005).
Glazener, C. M. A. et al. Conservative management of persistent postnatal urinary and faecal incontinence: randomised controlled trial. BMJ 323, 593–596 (2001).
MacArthur, C. et al. Persistent urinary incontinence and delivery mode history: a six-year longitudinal study. BJOG 113, 218–224 (2006).
MacArthur, C. et al. Urinary incontinence persisting after childbirth: extent, delivery history, and effects in a 12-year longitudinal cohort study. BJOG 123, 1022–1029 (2015).
MacArthur, C. et al. Obstetric practice and faecal incontinence three months after delivery. BJOG 108, 678–683 (2001).
Macarthur, C. et al. Faecal incontinence and mode of first and subsequent delivery: a six-year longitudinal study. BJOG 112, 1075–1082 (2005).
Macarthur, C. et al. Faecal incontinence persisting after childbirth: a 12 year longitudinal study. BJOG 120, 169–178 (2013).
Gutman, R. E. et al. Is there a pelvic organ prolapse threshold that predicts pelvic floor symptoms? Am. J. Obstet. Gynecol. 199, 683.e1–683.e7 (2008).
MacArthur, C. et al. Exclusive caesarean section delivery and subsequent urinary and faecal incontinence: a 12-year longitudinal study. BJOG 118, 1001–1007 (2011).
Glazener, C. et al. Childbirth and prolapse: long-term associations with the symptoms and objective measurement of pelvic organ prolapse. BJOG 120, 161–168 (2013).
Gyhagen, M. et al. The prevalence of urinary incontinence 20 years after childbirth: a national cohort study in singleton primiparae after vaginal or caesarean delivery. BJOG 120, 144–151 (2013).
Gyhagen, M. et al. Faecal incontinence 20 years after one birth: a comparison between vaginal delivery and caesarean section. Int. Urogynecol. J. 25, 1411–1418 (2014).
Gyhagen, M. et al. Prevalence and risk factors for pelvic organ prolapse 20 years after childbirth: a national cohort study in singleton primiparae after vaginal or caesarean delivery. BJOG 120, 152–160 (2013).
Gyhagen, M., Akervall, S. & Milsom, I. Clustering of pelvic floor disorders 20 years after one vaginal or one cesarean birth. Int. Urogynecol. J. 26, 1115–1121 (2015).
Gainey, H. Post-partum observation of pelvic tissue damage. Am. J. Obstet. Gynecol. 46, 457–466 (1943).
Gainey, H. L. Postpartum observation of pelvic tissue damage: further studies. Am. J. Obstet. Gynecol. 70, 800–807 (1955).
Miller, J. M. et al. Evaluating maternal recovery from labor and delivery: bone and levator ani injuries. Am. J. Obstet. Gynecol. 213, 188.e1–188.e11 (2015).
Chan, S. S. et al. Prevalence of levator ani muscle injury in Chinese women after first delivery. Ultrasound Obstet. Gynecol. 39, 704–709 (2012).
DeLancey, J. O. et al. The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet. Gynecol. 101, 46–53 (2003).
Dietz, H. P. & Wilson, P. D. Childbirth and pelvic floor trauma. Best Pract. Res. Clin. Obstet. Gynaecol. 19, 913–924 (2005).
Krofta, L. et al. Pubococcygeus-puborectalis trauma after forceps delivery: evaluation of the levator ani muscle with 3D/4D ultrasound. Int. Urogynecol. J. Pelvic Floor Dysfunct. 20, 1175–1181 (2009).
Shek, K. L. & Dietz, H. P. Intrapartum risk factors for levator trauma. BJOG 117, 1485–1492 (2010).
Dietz, H. P. Pelvic floor trauma following vaginal delivery. Curr. Opin. Obstet. Gynecol. 18, 528–537 (2006).
Falkert, A. et al. Three-dimensional ultrasound of pelvic floor: is there a correlation with delivery mode and persisting pelvic floor disorders 18–24 months after first delivery? Ultrasound Obstet. Gynecol. 41, 204–209 (2013).
Parente, M. P. et al. Deformation of the pelvic floor muscles during a vaginal delivery. Int. Urogynecol. J. Pelvic Floor Dysfunct. 19, 65–71 (2008).
Hoyte, L. et al. Quantity and distribution of levator ani stretch during simulated vaginal childbirth. Am. J. Obstet. Gynecol. 199, 198.e1–198.e5 (2008).
Brooks, S. V., Zerba, E. & Faulkner, J. A. Injury to muscle fibres after single stretches of passive and maximally stimulated muscles in mice. J. Physiol. 488, 459–469 (1995).
Lien, K. C. et al. Levator ani muscle stretch induced by simulated vaginal birth. Obstet. Gynecol. 103, 31–40 (2004).
Jing, D., Ashton-Miller, J. A. & DeLancey, J. O. A subject-specific anisotropic visco-hyperelastic finite element model of female pelvic floor stress and strain during the second stage of labor. J. Biomech. 45, 455–460 (2012).
Li, X. et al. Anisotropic effects of the levator ani muscle during childbirth. Biomech. Model. Mechanobiol. 10, 485–494 (2011).
Li, X. et al. Modelling childbirth: comparing athlete and non-athlete pelvic floor mechanics. Med. Image Comput. Comput. Assist Interv 11, 750–757 (2008).
Silva, M. E. et al. Study on the influence of the fetus head molding on the biomechanical behavior of the pelvic floor muscles, during vaginal delivery. J. Biomech. 48, 1600–1605 (2015).
Lien, K. C. et al. Pudendal nerve stretch during vaginal birth: a 3D computer simulation. Am. J. Obstet. Gynecol. 192, 1669–1676 (2005).
Robert, R. et al. Anatomic basis of chronic perineal pain: role of the pudendal nerve. Surg. Radiol. Anat. 20, 93–98 (1998).
Brown, R. et al. Effects of acute graded strain on efferent conduction properties in the rabbit tibial nerve. Clin. Orthop. Relat. Res. 288–294 (1993).
Snooks, S. J. et al. Injury to innervation of pelvic floor sphincter musculature in childbirth. Lancet 2, 546–550 (1984).
Allen, R. E. et al. Pelvic floor damage and childbirth: aneurophysiological study. Br. J. Obstet. Gynaecol. 97, 770–779 (1990).
Weidner, A. C. et al. Neuropathic injury to the levator ani occurs in 1 in 4 primiparous women. Am. J. Obstet. Gynecol. 195, 1851–1856 (2006).
South, M. M. et al. Levator ani denervation and reinnervation 6 months after childbirth. Am. J. Obstet. Gynecol. 200, 519.e1–519.e7 (2009).
Guillemeau, J. Les oeuvres de chirurgie de Jacques Guillemeau (N. Buon, 1612).
McPherson, K. C. et al. Can the risk of obstetric anal sphincter injuries (OASIs) be predicted using a risk-scoring system? BMC Res. Notes 7, 471 (2014).
Andrews, V. et al. Occult anal sphincter injuries — myth or reality? BJOG 113, 195–200 (2006).
Sultan, A. H., Thakar, R. & Fenner, D. E. Perineal and Anal Sphincter Trauma (Springer, 2007).
Oberwalder, M. et al. The association between late-onset fecal incontinence and obstetric anal sphincter defects. Arch. Surg. 139, 429–432 (2004).
Guzman Rojas, R. A. et al. Prevalence of anal sphincter injury in primiparous women. Ultrasound Obstet. Gynecol. 42, 461–466 (2013).
Sultan, A. H. et al. Anal-sphincter disruption during vaginal delivery. N. Engl. J. Med. 329, 1905–1911 (1993).
Rieger, N. et al. The effect of a normal vaginal delivery on anal function. Acta Obstet. Gynecol. Scand. 76, 769–772 (1997).
Chaliha, C. et al. Anal function: effect of pregnancy and delivery. Am. J. Obstet. Gynecol. 185, 427–432 (2001).
Willis, S. et al. Childbirth and incontinence: a prospective study on anal sphincter morphology and function before and early after vaginal delivery. Langenbecks Arch. Surg. 387, 101–107 (2002).
Dietz, H. P. et al. Does pregnancy affect pelvic organ mobility? Aust. N. Z. J. Obstet. Gynaecol. 44, 517–520 (2004).
Chan, S. S. et al. Pelvic floor biometry during a first singleton pregnancy and the relationship with symptoms of pelvic floor disorders: a prospective observational study. BJOG 121, 121–129 (2014).
van Veelen, A., Schweitzer, K. & van der Vaart, H. Ultrasound assessment of urethral support in women with stress urinary incontinence during and after first pregnancy. Obstet. Gynecol. 124, 249–256 (2014).
DeLancey, J. O. et al. Vaginal birth and de novo stress incontinence: relative contributions of urethral dysfunction and mobility. Obstet. Gynecol. 110, 354–362 (2007).
van Geelen, J. M. et al. The urethral pressure profile in≈pregnancy and after delivery in healthy nulliparous women. Am. J. Obstet. Gynecol. 144, 636–649 (1982).
Weidner, A. C. et al. Change in urethral sphincter neuromuscular function during pregnancy persists after delivery. Am J. Obstet. Gynecol. 201, 529.e1–529.e6 (2009).
Liu, S. et al. Maternal mortality and severe morbidity associated with low-risk planned cesarean delivery versus planned vaginal delivery at term. CMAJ 176, 455–460 (2007).
Deneux-Tharaux, C. et al. Postpartum maternal mortality and cesarean delivery. Obstet. Gynecol. 108, 541–548 (2006).
Nelson, R. L. et al. Cesarean delivery for the prevention of anal incontinence. Cochrane Database Syst. Rev. 2, CD006756 (2010).
Callewaert, G. et al. The impact of vaginal delivery on pelvic floor function — delivery as a time point for secondary prevention: a commentary. BJOG 123, 678–681 (2016).
Hsu, S. et al. A clinician's guide to the ABCs of cardiovascular disease prevention: the Johns Hopkins Ciccarone Center for the Prevention of Heart Disease and American College of Cardiology Cardiosource Approach to the Million Hearts Initiative. Clin. Cardiol. 36, 383–393 (2013).
Glazener, C. M. et al. Twelve-year follow-up of conservative management of postnatal urinary and faecal incontinence and prolapse outcomes: randomised controlled trial. BJOG 121, 112–120 (2014).
Enea, D. et al. Single-stage cartilage repair in the knee with microfracture covered with a resorbable polymer-based matrix and autologous bone marrow concentrate. Knee 20, 562–569 (2013).
Chase, L. G. & Vemuri, M. C. Mesenchymal Stem Cell Therapy (Springer, 2013).
Kuroda, R. et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage 15, 226–231 (2007).
Cannon, T. W. et al. Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology 62, 958–963 (2003).
Dissaranan, C. et al. Rat mesenchymal stem cell secretome promotes elastogenesis and facilitates recovery from simulated childbirth injury. Cell Transplant. 23, 1395–1406 (2014).
Salcedo, L. et al. Mesenchymal stem cells can improve anal pressures after anal sphincter injury. Stem Cell Res. 10, 95–102 (2013).
Lin, G. et al. Treatment of stress urinary incontinence with adipose tissue-derived stem cells. Cytotherapy 12, 88–95 (2010).
Kharraz, Y. et al. Macrophage plasticity and the role of inflammation in skeletal muscle repair. Mediators Inflamm. 2013, 491497 (2013).
Ley, K. et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 (2007).
Tidball, J. G. & Villalta, S. A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1173–R1187 (2010).
Nguyen, H. X. & Tidball, J. G. Interactions between neutrophils and macrophages promote macrophage killing of rat muscle cells in vitro. J. Physiol. 547, 125–132 (2003).
Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 3, 23–35 (2003).
Villalta, S. A. et al. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum. Mol. Genet. 18, 482–496 (2009).
Arnold, L. et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204, 1057–1069 (2007).
Yin, H., Price, F. & Rudnicki, M. A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 93, 23–67 (2013).
Madaro, L. & Bouche, M. From innate to adaptive immune response in muscular dystrophies and skeletal muscle regeneration: the role of lymphocytes. Biomed Res. Int. 2014, 438675 (2014).
Sampaolesi, M. et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature 444, 574–579 (2006).
Lolmede, K. et al. Inflammatory and alternatively activated human macrophages attract vessel-associated stem cells, relying on separate HMGB1- and MMP-9-dependent pathways. J. Leukoc. Biol. 85, 779–787 (2009).
Schiaffino, S. Skeletal Muscle Repair and Regeneration (Springer, 2008).
Tos, P. et al. Future perspectives in nerve repair and regeneration. Int. Rev. Neurobiol. 109, 165–192 (2013).
Perkins, N. M. & Tracey, D. J. Hyperalgesia due to nerve injury: role of neutrophils. Neuroscience 101, 745–757 (2000).
Mueller, M. et al. Macrophage response to peripheral nerve injury: the quantitative contribution of resident and hematogenous macrophages. Lab. Invest. 83, 175–185 (2003).
Faroni, A. et al. Peripheral nerve regeneration: experimental strategies and future perspectives. Adv. Drug Deliv. Rev. 82–83, 160–167 (2015).
Geuna, S. et al. Chapter 3: histology of the peripheral nerve and changes occurring during nerve regeneration. Int. Rev. Neurobiol. 87, 27–46 (2009).
Gaudet, A. D., Popovich, P. G. & Ramer, M. S. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J. Neuroinflammation 8, 110 (2011).
Ydens, E. et al. The neuroinflammatory role of Schwann cells in disease. Neurobiol. Dis. 55, 95–103 (2013).
Ydens, E. et al. Acute injury in the peripheral nervous system triggers an alternative macrophage response. J. Neuroinflammation 9, 176 (2012).
Hannan, J. L. et al. Temporal changes in neurotrophic factors and neurite outgrowth in the major pelvic ganglion following cavernous nerve injury. J. Neurosci. Res. 93, 954–963 (2015).
Scheib, J. & Hoke, A. Advances in peripheral nerve regeneration. Nat. Rev. Neurol. 9, 668–676 (2013).
Hanz, S. et al. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron 40, 1095–1104 (2003).
Wujek, J. R. & Lasek, R. J. Correlation of axonal regeneration and slow component B in two branches of a single axon. J. Neurosci. 3, 243–251 (1983).
Veltri, K. et al. Contribution of the distal nerve sheath to nerve and muscle preservation following denervation and sensory protection. J. Reconstr. Microsurg. 21, 57–70 (2005).
Viguie, C. A. et al. Quantitative study of the effects of long-term denervation on the extensor digitorum longus muscle of the rat. Anat. Rec. 248, 346–354 (1997).
Moalli, P. A. et al. A rat model to study the structural properties of the vagina and its supportive tissues. Am. J. Obstet. Gynecol. 192, 80–88 (2005).
Do Carmo, E. et al. Histochemical and morphological characteristics of the levator ani muscle in rats. Int. J. Morphol. 4, 1195–1201 (2011).
Poortmans, A. & Wyndaele, J. J. M. levator ani in the rat: does it really lift the anus? Anat. Rec. 251, 20–27 (1998).
Abramowitch, S. D. et al. Tissue mechanics, animal models, and pelvic organ prolapse: a review. Eur. J. Obstet. Gynecol. Reprod. Biol. 144 (Suppl. 1), S146–S158 (2009).
Couri, B. M. et al. Animal models of female pelvic organ prolapse: lessons learned. Expert Rev. Obstet. Gynecol. 7, 249–260 (2012).
Chermansky, C. J. et al. A model of intrinsic sphincteric deficiency in the rat: electrocauterization. Neurourol. Urodyn. 23, 166–171 (2004).
Eberli, D. et al. A canine model of irreversible urethral sphincter insufficiency. BJU Int. 103, 248–253 (2009).
Zutshi, M. et al. Effects of sphincterotomy and pudendal nerve transection on the anal sphincter in a rat model. Dis. Colon Rectum. 52, 1321–1329 (2009).
Wai, C. Y. et al. Recovery of external anal sphincter contractile function after prolonged vaginal distention or sphincter transection in an animal model. Obstet. Gynecol. 111, 1426–1434 (2008).
Rodriguez, L. V. et al. New objective measures to quantify stress urinary incontinence in a novel durable animal model of intrinsic sphincter deficiency. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R1332–R1338 (2005).
Kefer, J. C., Liu, G. & Daneshgari, F. Pubo-urethral ligament injury causes long-term stress urinary incontinence in female rats: an animal model of the integral theory. J. Urol. 181, 397–400 (2009).
Conway, D. A. et al. Comparison of leak point pressure methods in an animal model of stress urinary incontinence. Int. Urogynecol. J. Pelvic Floor Dysfunct. 16, 359–363 (2005).
Hijaz, A. et al. Animal models of female stress urinary incontinence. J. Urol. 179, 2103–2110 (2008).
Peng, C. W. et al. External urethral sphincter activity in a rat model of pudendal nerve injury. Neurourol. Urodyn. 25, 388–396 (2006).
Healy, C. F. et al. Experimental models of neuropathic fecal incontinence: an animal model of childbirth injury to the pudendal nerve and external anal sphincter. Dis. Colon Rectum. 51, 1619–1626 (2008).
Wang, G. et al. Effects of prolonged vaginal distension and beta-aminopropionitrile on urinary continence and urethral structure. Urology 78, 968.e13–968.e19 (2011).
Wood, H. M. et al. Cytokine expression after vaginal distention of different durations in virgin Sprague-Dawley rats. J. Urol. 180, 753–759 (2008).
Damaser, M. S. et al. Effect of vaginal distension on blood flow and hypoxia of urogenital organs of the female rat. J. Appl. Physiol. 98, 1884–1890 (2005).
Damaser, M. S. et al. Functional and neuroanatomical effects of vaginal distention and pudendal nerve crush in the female rat. J. Urol. 170, 1027–1031 (2003).
Hong, S. H. et al. Comparison of three types of stress urinary incontinence rat models: electrocauterization, pudendal denervation, and vaginal distension. Urology 81, 465.e1–465.e6 (2013).
Jiang, H. H. et al. Dual simulated childbirth injuries result in slowed recovery of pudendal nerve and urethral function. Neurourol. Urodyn. 28, 229–235 (2009).
Pan, H. Q. et al. Increased duration of simulated childbirth injuries results in increased time to recovery. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R1738–R1744 (2007).
Woo, L. L. et al. Over expression of stem cell homing cytokines in urogenital organs following vaginal distention. J. Urol. 177, 1568–1572 (2007).
Cannon, T. W. & Damaser, M. S. Effects of anesthesia on cystometry and leak point pressure of the female rat. Life Sci. 69, 1193–1202 (2001).
Hakim, L. et al. High-frequency micro-ultrasound: a novel method to assess external urethral sphincter function in rats following simulated birth injury. Neurourol. Urodyn. 34, 264–269 (2014).
Pan, H. Q. et al. Dual simulated childbirth injury delays anatomic recovery. Am. J. Physiol. Renal Physiol. 296, F277–F283 (2009).
Salem, H. K. & Thiemermann, C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells 28, 585–596 (2010).
Uccelli, A., Moretta, L. & Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 8, 726–736 (2008).
Pittenger, M. F. et al. Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 (1999).
Petersen, B. E. et al. Bone marrow as a potential source of hepatic oval cells. Science 284, 1168–1170 (1999).
Kopen, G. C., Prockop, D. J. & Phinney, D. G. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc. Natl Acad. Sci. USA 96, 10711–10716 (1999).
Jori, F. P. et al. Molecular pathways involved in neural in vitro differentiation of marrow stromal stem cells. J. Cell. Biochem. 94, 645–655 (2005).
Dellavalle, A. et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat. Commun. 2, 499 (2011).
Dimarino, A. M., Caplan, A. I. & Bonfield, T. L. Mesenchymal stem cells in tissue repair. Front. Immunol. 4, 201 (2013).
Albersen, M. et al. Expression of a distinct set of chemokine receptors in adipose tissue-derived stem cells is responsible for in vitro migration toward chemokines appearing in the major pelvic ganglion following cavernous nerve injury. Sex. Med. 1, 3–15 (2013).
Chapel, A. et al. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J. Gene Med. 5, 1028–1038 (2003).
Salcedo, L. et al. Chemokine upregulation in response to anal sphincter and pudendal nerve injury: potential signals for stem cell homing. Int. J. Colorectal Dis. 26, 1577–1581 (2011).
Nemeth, K. et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 15, 42–49 (2009).
Raffaghello, L. et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells 26, 151–162 (2008).
Kim, J. & Hematti, P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp. Hematol. 37, 1445–1453 (2009).
Cutler, A. J. et al. Umbilical cord-derived mesenchymal stromal cells modulate monocyte function to suppress T cell proliferation. J. Immunol. 185, 6617–6623 (2010).
Maggini, J. et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS ONE 5, e9252 (2010).
Zhang, Q. Z. et al. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells 28, 1856–1868 (2010).
Munoz, J. R. et al. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc. Natl Acad. Sci. USA 102, 18171–18176 (2005).
Heldring, N. et al. Therapeutic potential of multipotent mesenchymal stromal cells and their extracellular vesicles. Hum. Gene Ther. 26, 506–517 (2015).
Lee, R. H. et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5, 54–63 (2009).
Verhoekx, J. S. et al. Adipose-derived stem cells inhibit the contractile myofibroblast in Dupuytren's disease. Plast. Reconstr. Surg. 132, 1139–1148 (2013).
Semedo, P. et al. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells 27, 3063–3073 (2009).
Ortiz, L. A. et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc. Natl Acad. Sci. USA 100, 8407–8411 (2003).
Zhao, W. et al. Intravenous injection of mesenchymal stem cells is effective in treating liver fibrosis. World J. Gastroenterol. 18, 1048–1058 (2012).
Yiou, R. et al. Muscle precursor cell autografting in a murine model of urethral sphincter injury. BJU Int. 89, 298–302 (2002).
Lee, J. Y. et al. The effects of periurethral muscle-derived stem cell injection on leak point pressure in a rat model of stress urinary incontinence. Int. Urogynecol. J. Pelvic Floor Dysfunct. 14, 31–37 (2003).
Kwon, D. et al. Periurethral cellular injection: comparison of muscle-derived progenitor cells and fibroblasts with regard to efficacy and tissue contractility in an animal model of stress urinary incontinence. Urology 68, 449–454 (2006).
Chermansky, C. J. et al. Intraurethral muscle-derived cell injections increase leak point pressure in a rat model of intrinsic sphincter deficiency. Urology 63, 780–785 (2004).
Cruz, M. et al. Pelvic organ distribution of mesenchymal stem cells injected intravenously after simulated childbirth injury in female rats. Obstet. Gynecol. Int. 2012, 612946 (2012).
Deng, K. et al. Mesenchymal stem cells and their secretome preserve nerve and urethral function in a dual muscle and nerve injury stress urinary incontinence model. Am. J. Physiol. Renal Physiol. 308, F92–F100 (2015).
Salcedo, L. et al. Functional outcome after anal sphincter injury and treatment with mesenchymal stem cells. Stem Cells Transl Med. 3, 760–767 (2014).
Carr, L. K. et al. 1-year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. Int. Urogynecol. J. Pelvic Floor Dysfunct. 19, 881–883 (2008).
Carr, L. K. et al. Autologous muscle derived cell therapy for stress urinary incontinence: a prospective, dose ranging study. J. Urol. 189, 595–601 (2013).
Peters, K. M. et al. Autologous muscle derived cells for treatment of stress urinary incontinence in women. J. Urol. 192, 469–476 (2014).
Sebe, P. et al. Intrasphincteric injections of autologous muscular cells in women with refractory stress urinary incontinence: a prospective study. Int. Urogynecol. J. 22, 183–189 (2011).
Gras, S., Klarskov, N. & Lose, G. Intraurethral injection of autologous minced skeletal muscle: a simple surgical treatment for stress urinary incontinence. J. Urol. 192, 850–855 (2014).
Lee, C. N. et al. Human cord blood stem cell therapy for treatment of stress urinary incontinence. J. Korean Med. Sci. 25, 813–816 (2010).
Yamamoto, T. et al. Periurethral injection of autologous adipose-derived regenerative cells for the treatment of male stress urinary incontinence: report of three initial cases. Int. J. Urol. 19, 652–659 (2012).
Frudinger, A. et al. Muscle-derived cell injection to treat anal incontinence due to obstetric trauma: pilot study with 1 year follow-up. Gut 59, 55–61 (2010).
Frudinger, A. et al. Autologous skeletal-muscle-derived cell injection for anal incontinence due to obstetric trauma: a 5-year follow-up of an initial study of 10 patients. Colorectal Dis. 17, 794–801 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01382602 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01600755 (2016).
International Society for Stem Cell Research. Guidelines for the Clinical Translation of Stem Cells [online] (2008).
Author information
Authors and Affiliations
Contributions
G.C. and N.S. researched data for this article, G.C., M.S., M.A. and J.D. made a substantial contribution to discussions of content, G.C., M.M.C.M.d.C., N.S., M.A. and J.D. wrote the manuscript and all authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S2 (table)
Use of mesenchymal stem cells in rat model of simulated vaginal birth injury (DOC 205 kb)
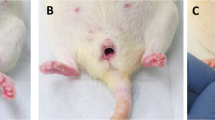
Trauma to the levator ani muscle during childbirth
Exposure of the levator ani to excessive forces during childbirth, which substantially increase the risk of pelvic-floor dysfunctions. (MP4 4656 kb)
Rights and permissions
About this article
Cite this article
Callewaert, G., Da Cunha, M., Sindhwani, N. et al. Cell-based secondary prevention of childbirth-induced pelvic floor trauma. Nat Rev Urol 14, 373–385 (2017). https://doi.org/10.1038/nrurol.2017.42
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2017.42
This article is cited by
-
VEGF overexpressed mesoangioblasts enhance urethral and vaginal recovery following simulated vaginal birth in rats
Scientific Reports (2023)
-
Obstetric risk factors for anorectal dysfunction after delivery: a systematic review and meta-analysis
International Urogynecology Journal (2021)
-
Signs of damage in pelvic floor muscles at the end of pregnancy in rabbits
International Urogynecology Journal (2019)
-
Fate of mesoangioblasts in a vaginal birth injury model: influence of the route of administration
Scientific Reports (2018)