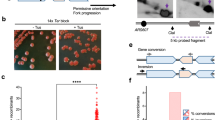
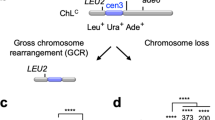
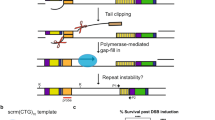
Abstract
Several molecular mechanisms have been proposed to explain trinucleotide repeat expansions. Here we show that in yeast srs2Δ cells, CTG repeats undergo both expansions and contractions, and they show increased chromosomal fragility. Deletion of RAD52 or RAD51 suppresses these phenotypes, suggesting that recombination triggers trinucleotide repeat instability in srs2Δ cells. In sgs1Δ cells, CTG repeats undergo contractions and increased fragility by a mechanism partially dependent on RAD52 and RAD51. Analysis of replication intermediates revealed abundant joint molecules at the CTG repeats during S phase. These molecules migrate similarly to reversed replication forks, and their presence is dependent on SRS2 and SGS1 but not RAD51. Our results suggest that Srs2 promotes fork reversal in repetitive sequences, preventing repeat instability and fragility. In the absence of Srs2 or Sgs1, DNA damage accumulates and is processed by homologous recombination, triggering repeat rearrangements.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
26 January 2009
In the version of this article initially published online, the labels in Figure 1c, in the key to Figure 4 and in Table 2 were incorrect. The error has been corrected for the print, PDF and HTML versions of this article.
References
Gatchel, J.R. & Zoghbi, H.Y. Diseases of unstable repeat expansion: mechanisms and common principles. Nat. Rev. Genet. 6, 743–755 (2005).
Mirkin, S.M. DNA structures, repeat expansions and human hereditary disorders. Curr. Opin. Struct. Biol. 16, 351–358 (2006).
Lenzmeier, B.A. & Freudenreich, C.H. Trinucleotide repeat instability: a hairpin curve at the crossroads of replication, recombination, and repair. Cytogenet. Genome Res. 100, 7–24 (2003).
Pearson, C.E., Edamura, K.N. & Cleary, J.D. Repeat instability: mechanisms of dynamic mutations. Nat. Rev. Genet. 6, 729–742 (2005).
Richard, G.-F., Dujon, B. & Haber, J.E. Double-strand break repair can lead to high frequencies of deletions within short CAG/CTG trinucleotide repeats. Mol. Gen. Genet. 261, 871–882 (1999).
Richard, G.-F., Cyncynatus, C. & Dujon, B. Contractions and expansions of CAG/CTG trinucleotide repeats occur during ectopic gene conversion in yeast, by a MUS81-independent mechanism. J. Mol. Biol. 326, 769–782 (2003).
Richard, G.-F., Goellner, G.M., McMurray, C.T. & Haber, J.E. Recombination-induced CAG trinucleotide repeat expansions in yeast involve the MRE11/RAD50/XRS2 complex. EMBO J. 19, 2381–2390 (2000).
Richard, G.-F. & Pâques, F. Mini- and microsatellite expansions: the recombination connection. EMBO Rep. 1, 122–126 (2000).
Freudenreich, C.H., Kantrow, S.M. & Zakian, V.A. Expansion and length-dependent fragility of CTG repeats in yeast. Science 279, 853–856 (1998).
Spiro, C. et al. Inhibition of FEN-1 processing by DNA secondary structure at trinucleotide repeats. Mol. Cell 4, 1079–1085 (1999).
Schweitzer, J.K. & Livingston, D.M. Expansions of CAG repeat tracts are frequent in a yeast mutant defective in Okazaki fragment maturation. Hum. Mol. Genet. 7, 69–74 (1998).
Loeillet, S. et al. Genetic network interactions among replication, repair and nuclear pore deficiencies in yeast. DNA Repair (Amst.) 4, 459–468 (2005).
Gangloff, S., McDonald, J.P., Bendixen, C., Arthur, L. & Rothstein, R. The yeast type I topoisomerase Top3 interacts with Sgs1, a DNA helicase homolog: a potential eukaryotic reverse gyrase. Mol. Cell. Biol. 14, 8391–8398 (1994).
Watt, P.M., Hickson, I.D., Borts, R.H. & Louis, E.J. SGS1, a homologue of the Bloom's and Werner's syndrome genes, is required for maintenance of genome stability in Saccharomyces cerevisiae. Genetics 144, 935–945 (1996).
Richard, G.-F., Kerrest, A., Lafontaine, I. & Dujon, B. Comparative genomics of hemiascomycete yeasts: genes involved in DNA replication, repair, and recombination. Mol. Biol. Evol. 22, 1011–1023 (2005).
Wu, L. & Hickson, I.D. The Bloom's syndrome helicase suppresses crossing over during homologous recombination. Nature 426, 870–874 (2003).
Ira, G., Malkova, A., Liberi, G., Foiani, M. & Haber, J.E. Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast. Cell 115, 401–411 (2003).
Robert, T., Dervins, D., Fabre, F. & Gangloff, S. Mrc1 and Srs2 are major actors in the regulation of spontaneous crossover. EMBO J. 25, 2837–2846 (2006).
Adams, M.D., McVey, M. & Sekelsky, J.J. Drosophila BLM in double-strand break repair by synthesis-dependent strand annealing. Science 299, 265–267 (2003).
Aboussekhra, A. et al. RADH, a gene of Saccharomyces cerevisiae encoding a putative DNA helicase involved in DNA repair. Characteristics of radH mutants and sequence of the gene. Nucleic Acids Res. 17, 7211–7219 (1989).
Rong, L. & Klein, H.L. Purification and characterization of the SRS2 DNA helicase of the yeast Saccharomyces cerevisiae. J. Biol. Chem. 268, 1252–1259 (1993).
Krejci, L. et al. DNA helicase Srs2 disrupts the Rad51 presynaptic filament. Nature 423, 305–309 (2003).
Veaute, X. et al. The Srs2 helicase prevents recombination by disrupting Rad51 nucleoprotein filaments. Nature 423, 309–312 (2003).
Dupaigne, P. et al. The Srs2 helicase activity is stimulated by Rad51 filaments on dsDNA: implications for crossover incidence during mitotic recombination. Mol. Cell 29, 243–254 (2008).
Bhattacharyya, S. & Lahue, R.S. Saccharomyces cerevisiae Srs2 DNA helicase selectively blocks expansions of trinucleotide repeats. Mol. Cell. Biol. 24, 7324–7330 (2004).
Callahan, J.L., Andrews, K.J., Zakian, V.A. & Freudenreich, C.H. Mutations in yeast replication proteins that increase CAG/CTG expansions also increase repeat fragility. Mol. Cell. Biol. 23, 7849–7860 (2003).
Napierala, M., Parniewski, P., Pluciennik, A. & Wells, R.D. Long CTG•CAG repeat sequences markedly stimulate intramolecular recombination. J. Biol. Chem. 277, 34087–34100 (2002).
Pluciennik, A. et al. Long CTG•CAG repeats from myotonic dystrophy are preferred sites for intermolecular recombination. J. Biol. Chem. 277, 34074–34086 (2002).
Jankowski, C., Nasar, F. & Nag, D.K. Meiotic instability of CAG repeat tracts occurs by double-strand break repair in yeast. Proc. Natl. Acad. Sci. USA 97, 2134–2139 (2000).
Gangloff, S., Soustelle, C. & Fabre, F. Homologous recombination is responsible for cell death in the absence of the Sgs1 and Srs2 helicases. Nat. Genet. 25, 192–194 (2000).
Fabre, F., Chan, A., Heyer, W.-D. & Gangloff, S. Alternate pathways involving Sgs1/Top3, Mus81/Mms4, and Srs2 prevent formation of toxic recombination intermediates from single-stranded gaps created by DNA replication. Proc. Natl. Acad. Sci. USA 99, 16887–16892 (2002).
Freudenreich, C.H., Stavenhagen, J.B. & Zakian, V.A. Stability of a CTG/CAG trinucleotide repeat in yeast is dependent on its orientation in the genome. Mol. Cell. Biol. 17, 2090–2098 (1997).
Kang, S., Jaworski, A., Ohshima, K. & Wells, R.D. Expansion and deletion of CTG repeats from human disease genes are determined by the direction of replication in E. coli. Nat. Genet. 10, 213–217 (1995).
Zahra, R., Blackwood, J.K., Sales, J. & Leach, D.R.F. Proofreading and secondary structure processing determine the orientation dependence of CAG•CTG trinucleotide repeat instability in Escherichia coli. Genetics 176, 27–41 (2007).
Miret, J.J., Passoa-Brandaão, L. & Lahue, R.S. Orientation-dependent and sequence-specific expansions of CTG/CAG trinucleotide repeats in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 95, 12438–12443 (1998).
Maurer, D.J., O'Callaghan, B.L. & Livingston, D.M. Orientation dependence of trinucleotide CAG repeat instability in Saccharomyces cerevisiae. Mol. Cell. Biol. 16, 6617–6622 (1996).
Alvino, G.M. et al. Replication in hydroxyurea: it's a matter of time. Mol. Cell. Biol. 27, 6396–6406 (2007).
Lammers, M. & Follmann, H. Deoxyribonucleotide biosynthesis in yeast (Saccharomyces cerevisiae). A ribonucleotide reductase system of sufficient activity for DNA synthesis. Eur. J. Biochem. 140, 281–287 (1984).
Bhattacharyya, S. & Lahue, R.S. Srs2 helicase of Saccharomyces cerevisiae selectively unwinds triplet repeat DNA. J. Biol. Chem. 280, 33311–33317 (2005).
Lopes, M., Cotta-Ramusino, C., Liberi, G. & Foiani, M. Branch migrating sister chromatid junctions form at replication origins through Rad51/Rad52-independent mechanisms. Mol. Cell 12, 1499–1510 (2003).
Flores, M.J., Sanchez, N. & Michel, B. A fork-clearing role for UvrD. Mol. Microbiol. 57, 1664–1675 (2005).
Daee, D.L., Mertz, T. & Lahue, R.S. Postreplication repair inhibits CAG-CTG repeat expansions in Saccharomyces cerevisiae. Mol. Cell. Biol. 27, 102–110 (2007).
Goldfarb, T. & Alani, E. Distinct roles for the Saccharomyces cerevisiae mismatch repair proteins in heteroduplex rejection, mismatch repair and nonhomologous tail removal. Genetics 169, 563–574 (2005).
Sugawara, N., Goldfarb, T., Studamire, B., Alani, E. & Haber, J.E. Heteroduplex rejection during single-strand annealing requires Sgs1 helicase and mismatch repair proteins Msh2 and Msh6 but not Pms1. Proc. Natl. Acad. Sci. USA 101, 9315–9320 (2004).
Richard, G.F., Kerrest, A. & Dujon, B. Comparative genomics and molecular dynamics of DNA repeats in eukaryotes. Microbiol. Mol. Biol. Rev. 72, 686–727 (2008).
Kamath-Loeb, A.S., Johansson, E., Burgers, P.M. & Loeb, L.A. Functional interaction between the Werner Syndrome protein and DNA polymerase δ. Proc. Natl. Acad. Sci. USA 97, 4603–4608 (2000).
Kamath-Loeb, A.S., Loeb, L.A., Johansson, E., Burgers, P.M. & Fry, M. Interactions between the Werner syndrome helicase and DNA polymerase δ specifically facilitate copying of tetraplex and hairpin structures of the d(CGG)n trinucleotide repeat sequence. J. Biol. Chem. 276, 16439–16446 (2001).
Pelletier, R., Krasilnikova, M.M., Samadashwily, G.M., Lahue, R. & Mirkin, S.M. Replication and expansion of trinucleotide repeats in yeast. Mol. Cell. Biol. 23, 1349–1357 (2003).
Samadashwily, G.M., Raca, G. & Mirkin, S.M. Trinucleotide repeats affect DNA replication in vivo. Nat. Genet. 17, 298–304 (1997).
Lopes, M. et al. The DNA replication checkpoint response stabilizes stalled replication forks. Nature 412, 557–561 (2001).
Long, D.T. & Kreuzer, K.N. Regression supports two mechanisms of fork processing in phage T4. Proc. Natl. Acad. Sci. USA 105, 6852–6857 (2008).
Fouché, N., Özgür, S., Roy, D. & Griffith, J.D. Replication fork regression in repetitive DNAs. Nucleic Acids Res. 34, 6044–6050 (2006).
Fierro-Fernandez, M., Hernandez, P., Krimer, D.B. & Schvartzman, J.B. Replication fork reversal occurs spontaneously after digestion but is constrained in supercoiled domains. J. Biol. Chem. 282, 18190–18196 (2007).
Fierro-Fernandez, M., Hernandez, P., Krimer, D.B. & Schvartzman, J.B. Topological locking restrains replication fork reversal. Proc. Natl. Acad. Sci. USA 104, 1500–1505 (2007).
Liberi, G. et al. Rad51-dependent DNA structures accumulate at damaged replication forks in sgs1 mutants defective in the yeast ortholog of BLM RecQ helicase. Genes Dev. 19, 339–350 (2005).
Cobb, J.A., Bjergbaek, L., Shimada, K., Frei, C. & Gasser, S.M. DNA polymerase stabilization at stalled replication forks requires Mec1 and the RecQ helicase Sgs1. EMBO J. 22, 4325–4336 (2003).
Nieduszynski, C.A., Knox, Y. & Donaldson, A.D. Genome-wide identification of replication origins in yeast by comparative genomics. Genes Dev. 20, 1874–1879 (2006).
Raghuraman, M.K. et al. Replication dynamics of the yeast genome. Science 294, 115–121 (2001).
Liberi, G. et al. Methods to study replication fork collapse in budding yeast. Methods Enzymol. 409, 442–462 (2006).
Goldfless, S.J., Morag, A.S., Belisle, K.A., Sutera, V.A.J. & Lovett, S.T. DNA repeat rearrangements mediated by DnaK-dependent replication fork repair. Mol. Cell 21, 595–604 (2006).
Acknowledgements
A.K. is grateful to G. Maffioletti for teaching her the two-dimensional gel electrophoresis technique and helping with her first successful experiments. A.K. and G.-F.R. thank the people who gave them advice concerning two-dimensional gel electrophoresis: M. Foiani, C. Maric, A. Ceschia and A. Kaykov. They also gratefully acknowledge the help of G. Millot for advice concerning the various statistical tests used in this manuscript. They and B.D. also thank their colleagues of the Unité de Génétique Moléculaire des Levures for many fruitful discussions and G. Fischer for careful reading of the manuscript. R.P.A. would like to acknowledge the help of K. Suryanarayanan in installing the SALVADOR program. A.K. was funded by the Ministère de la Recherche and the Fondation pour la Recherche Médicale (FRM). This work was supported by grant 3738 from the Association pour la Recherche contre le Cancer (ARC), grant ANR-05-BLAN-0331 from the Agence Nationale de la Recherche, US National Institutes of Health grant GM063066 to C.H.F., Tufts University FRAC award to C.H.F. and GSC Research Award to R.P.A. B.D. is a member of the Institut Universitaire de France.
Author information
Authors and Affiliations
Contributions
A.K. and G.-F.R. conceived of and performed the instability and two-dimensional studies on yeast chromosome X; C.H.F. and R.P.A. conceived of and performed the instability and fragility studies on the YAC, with R.S. contributing the rad51Δ (CAG)0 and (CAG)70 fragility analyses; R.B. and G.L. gave expert assistance with two-dimensional gel electrophoresis; A.K., B.D., G.-F.R., C.H.F. and R.P.A. analyzed the data and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1 and 2 (PDF 300 kb)
Rights and permissions
About this article
Cite this article
Kerrest, A., Anand, R., Sundararajan, R. et al. SRS2 and SGS1 prevent chromosomal breaks and stabilize triplet repeats by restraining recombination. Nat Struct Mol Biol 16, 159–167 (2009). https://doi.org/10.1038/nsmb.1544
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1544
This article is cited by
-
Dynamic alternative DNA structures in biology and disease
Nature Reviews Genetics (2023)
-
RNA biology of disease-associated microsatellite repeat expansions
Acta Neuropathologica Communications (2017)
-
Interrogating the “unsequenceable” genomic trinucleotide repeat disorders by long-read sequencing
Genome Medicine (2017)
-
The role of break-induced replication in large-scale expansions of (CAG)n/(CTG)n repeats
Nature Structural & Molecular Biology (2017)
-
Break-induced replication: an unhealthy choice for stress relief?
Nature Structural & Molecular Biology (2017)