Abstract
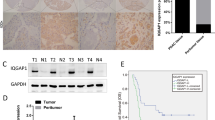
The Krüppel-like zinc-finger protein GLI1 functions as a downstream transcription factor of Hedgehog signaling and plays a pivotal role in the cellular proliferation of many types of tumors, including pancreatic ductal adenocarcinoma (PDA). PDA develops from dysplastic lesions called pancreatic intraepithelial neoplasia (PanIN) through a multistep carcinogenesis process that changes its cellular characteristics, including a mucin expression profile. Increased expression of a gel-forming mucin, MUC5AC, was previously revealed as a major biomarker for the poor prognosis of PDA patients, but the molecular mechanisms responsible for its expression and correlation with poor prognosis are not fully understood. Here we show that MUC5AC is a direct transcriptional target of GLI1 in PDA cells. Overexpression of GLI1 enhanced MUC5AC expression, and a double knockdown of GLI1 and GLI2 suppressed endogenous MUC5AC expression in PDA cells. Luciferase reporter assays revealed that GLI1 and GLI2 can activate the MUC5AC promoter through its conserved CACCC-box-like cis-regulatory elements. We also found that GLI1-upregulated MUC5AC was expressed in the intercellular junction between cultured PDA cells and interfered with the membrane localization of E-cadherin, leading to decreased E-cadherin-dependent cell–cell adhesion and promoting the migration and invasion of PDA cells. Consistently, GLI1 induced the nuclear accumulation and target gene expression of β-catenin in a MUC5AC-dependent manner. Finally, immunohistochemical analysis revealed that GLI1 expression statistically correlated with MUC5AC expression and also with altered subcellular localization of E-cadherin and β-catenin in PanIN lesions and PDA. This evidence revealed a new aspect of GLI1 function in modulating E-cadherin/β-catenin-regulated cancer cell properties through the expression of a gel-forming mucin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Agren M, Kogerman P, Kleman MI, Wessling M, Toftgard R . (2004). Expression of the PTCH1 tumor suppressor gene is regulated by alternative promoters and a single functional Gli-binding site. Gene 330: 101–114.
Aishima S, Kuroda Y, Nishihara Y, Taguchi K, Taketomi A, Maehara Y et al. (2006). Gastric mucin phenotype defines tumour progression and prognosis of intrahepatic cholangiocarcinoma: gastric foveolar type is associated with aggressive tumour behaviour. Histopathology 49: 35–44.
Al-Aynati MM, Radulovich N, Riddell RH, Tsao MS . (2004). Epithelial-cadherin and beta-catenin expression changes in pancreatic intraepithelial neoplasia. Clin Cancer Res 10: 1235–1240.
Bafna S, Kaur S, Batra SK . (2010). Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene 29: 2893–2904.
Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K et al. (2003). Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature 425: 846–851.
Boonla C, Wongkham S, Sheehan JK, Wongkham C, Bhudhisawasdi V, Tepsiri N et al. (2003). Prognostic value of serum MUC5AC mucin in patients with cholangiocarcinoma. Cancer 98: 1438–1443.
Briggs CD, Neal CP, Mann CD, Steward WP, Manson MM, Berry DP . (2009). Prognostic molecular markers in cholangiocarcinoma: a systematic review. Eur J Cancer 45: 33–47.
Eichberger T, Sander V, Schnidar H, Regl G, Kasper M, Schmid C et al. (2006). Overlapping and distinct transcriptional regulator properties of the GLI1 and GLI2 oncogenes. Genomics 87: 616–632.
Gkretsi V, Zhang Y, Tu Y, Chen K, Stolz DB, Yang Y et al. (2005). Physical and functional association of migfilin with cell-cell adhesions. J Cell Sci 118: 697–710.
Hollingsworth MA, Swanson BJ . (2004). Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer 4: 45–60.
Jepson S, Komatsu M, Haq B, Arango ME, Huang D, Carraway CA et al. (2002). Muc4/sialomucin complex, the intramembrane ErbB2 ligand, induces specific phosphorylation of ErbB2 and enhances expression of p27(kip), but does not activate mitogen-activated kinase or protein kinaseB/Akt pathways. Oncogene 21: 7524–7532.
Kasai K, Takahashi M, Osumi N, Sinnarajah S, Takeo T, Ikeda H et al. (2004). The G12 family of heterotrimeric G proteins and Rho GTPase mediate Sonic hedgehog signalling. Genes Cells 9: 49–58.
Kasai K, Inaguma S, Yoneyama A, Yoshikawa K, Ikeda H . (2008). SCL/TAL1 interrupting locus derepresses GLI1 from the negative control of suppressor-of-fused in pancreatic cancer cell. Cancer Res 68: 7723–7729.
Kasper M, Schnidar H, Neill GW, Hanneder M, Klingler S, Blaas L et al. (2006). Selective modulation of Hedgehog/GLI target gene expression by epidermal growth factor signaling in human keratinocytes. Mol Cell Biol 26: 6283–6298.
Lee HS, Lee HK, Kim HS, Yang HK, Kim YI, Kim WH . (2001). MUC1, MUC2, MUC5AC, and MUC6 expressions in gastric carcinomas: their roles as prognostic indicators. Cancer 92: 1427–1434.
Li D, Gallup M, Fan N, Szymkowski DE, Basbaum CB . (1998). Cloning of the amino-terminal and 5′-flanking region of the human MUC5AC mucin gene and transcriptional up-regulation by bacterial exoproducts. J Biol Chem 273: 6812–6820.
Nolan-Stevaux O, Lau J, Truitt ML, Chu GC, Hebrok M, Fernandez-Zapico ME et al. (2009). GLI1 is regulated through Smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev 23: 24–36.
Park ET, Gum JR, Kakar S, Kwon SW, Deng G, Kim YS . (2008). Aberrant expression of SOX2 upregulates MUC5AC gastric foveolar mucin in mucinous cancers of the colorectum and related lesions. Int J Cancer 122: 1253–1260.
Pasca di Magliano M, Sekine S, Ermilov A, Ferris J, Dlugosz AA, Hebrok M . (2006). Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes Dev 20: 3161–3173.
Prasad NB, Biankin AV, Fukushima N, Maitra A, Dhara S, Elkahloun AG et al. (2005). Gene expression profiles in pancreatic intraepithelial neoplasia reflect the effects of Hedgehog signaling on pancreatic ductal epithelial cells. Cancer Res 65: 1619–1626.
Sanada Y, Yoshida K, Ohara M, Oeda M, Konishi K, Tsutani Y . (2006). Histopathologic evaluation of stepwise progression of pancreatic carcinoma with immunohistochemical analysis of gastric epithelial transcription factor SOX2: comparison of expression patterns between invasive components and cancerous or nonneoplastic intraductal components. Pancreas 32: 164–170.
Schroeder JA, Thompson MC, Gardner MM, Gendler SJ . (2001). Transgenic MUC1 interacts with epidermal growth factor receptor and correlates with mitogen-activated protein kinase activation in the mouse mammary gland. J Biol Chem 276: 13057–13064.
Senapati S, Sharma P, Bafna S, Roy HK, Batra SK . (2008). The MUC gene family: their role in the diagnosis and prognosis of gastric cancer. Histol Histopathol 23: 1541–1552.
Shimokawa T, Furukawa Y, Sakai M, Li M, Miwa N, Lin YM et al. (2003). Involvement of the FGF18 gene in colorectal carcinogenesis, as a novel downstream target of the beta-catenin/T-cell factor complex. Cancer Res 63: 6116–6120.
Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R et al. (1999). The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA 96: 5522–5527.
Singh AP, Senapati S, Ponnusamy MP, Jain M, Lele SM, Davis JS et al. (2008). Clinical potential of mucins in diagnosis, prognosis, and therapy of ovarian cancer. Lancet Oncol 9: 1076–1085.
Takikita M, Altekruse S, Lynch CF, Goodman MT, Hernandez BY, Green M et al. (2009). Associations between selected biomarkers and prognosis in a population-based pancreatic cancer tissue microarray. Cancer Res 69: 2950–2955.
Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY et al. (2003). Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature 425: 851–856.
Truant S, Bruyneel E, Gouyer V, De Wever O, Pruvot FR, Mareel M et al. (2003). Requirement of both mucins and proteoglycans in cell-cell dissociation and invasiveness of colon carcinoma HT-29 cells. Int J Cancer 104: 683–694.
Vincent A, Perrais M, Desseyn JL, Aubert JP, Pigny P, Van Seuningen I . (2007). Epigenetic regulation (DNA methylation, histone modifications) of the 11p15 mucin genes (MUC2, MUC5AC, MUC5B, MUC6) in epithelial cancer cells. Oncogene 26: 6566–6576.
Wen Y, Caffrey TC, Wheelock MJ, Johnson KR, Hollingsworth MA . (2003). Nuclear association of the cytoplasmic tail of MUC1 and beta-catenin. J Biol Chem 278: 38029–38039.
Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP et al. (2008). A paracrine requirement for hedgehog signalling in cancer. Nature 455: 406–410.
Yoon JW, Kita Y, Frank DJ, Majewski RR, Konicek BA, Nobrega MA et al. (2002). Gene expression profiling leads to identification of GLI1-binding elements in target genes and a role for multiple downstream pathways in GLI1-induced cell transformation. J Biol Chem 277: 5548–5555.
Yu CJ, Yang PC, Shun CT, Lee YC, Kuo SH, Luh KT . (1996). Overexpression of MUC5 genes is associated with early post-operative metastasis in non-small-cell lung cancer. Int J Cancer 69: 457–465.
Yu CJ, Shih JY, Lee YC, Shun CT, Yuan A, Yang PC . (2005). Sialyl Lewis antigens: association with MUC5AC protein and correlation with post-operative recurrence of non-small cell lung cancer. Lung Cancer 47: 59–67.
Acknowledgements
We thank Yukiko Matsubara, Motoyasu Takeuchi and Naoki Igari (Aichi Medical University) for their expert technical assistance. We also thank Dr Akiko Tamakoshi (Aichi Medical University) for advice on statistical analyses and Dr Masahide Takahashi (Nagoya University) for comments on the manuscript. This research was supported in part by a Grant-in-Aid for Scientific Research (C) (to KK) and for Young Scientists (B) (to SI) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Inaguma, S., Kasai, K. & Ikeda, H. GLI1 facilitates the migration and invasion of pancreatic cancer cells through MUC5AC-mediated attenuation of E-cadherin. Oncogene 30, 714–723 (2011). https://doi.org/10.1038/onc.2010.459
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.459
Keywords
This article is cited by
-
Chromosome-level Dinobdella ferox genome provided a molecular model for its specific parasitism
Parasites & Vectors (2023)
-
Hedgehog signaling in tissue homeostasis, cancers, and targeted therapies
Signal Transduction and Targeted Therapy (2023)
-
Mutant GNAS limits tumor aggressiveness in established pancreatic cancer via antagonizing the KRAS-pathway
Journal of Gastroenterology (2022)
-
Targeting hypoxic tumor microenvironment in pancreatic cancer
Journal of Hematology & Oncology (2021)
-
Unraveling mucin domains in cancer and metastasis: when protectors become predators
Cancer and Metastasis Reviews (2020)