Abstract
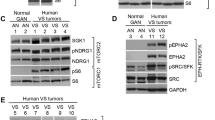
Neurofibromatosis type 2 (NF2) is caused by mutations in the NF2 gene that encodes a tumor-suppressor protein called merlin. NF2 is characterized by formation of multiple schwannomas, meningiomas and ependymomas. Merlin loss-of-function is associated with increased activity of Rac and p21-activated kinases (PAKs) and deregulation of cytoskeletal organization. LIM domain kinases (LIMK1 and 2) are substrate for Cdc42/Rac-PAK and modulate actin dynamics by phosphorylating cofilin at serine-3. This modification inactivates the actin severing and depolymerizing activity of cofilin. LIMKs also translocate into the nucleus and regulate cell cycle progression. Significantly, LIMKs are overexpressed in several tumor types, including skin, breast, lung, liver and prostate. Here we report that mouse Schwann cells (MSCs) in which merlin function is lost as a result of Nf2 exon2 deletion (Nf2ΔEx2) exhibited increased levels of LIMK1, LIMK2 and active phospho-Thr508/505-LIMK1/2, as well as phospho-Ser3-cofilin, compared with wild-type normal MSCs. Similarly, levels of LIMK1 and 2 total protein and active phosphorylated forms were elevated in human vestibular schwannomas compared with normal human Schwann cells (SCs). Reintroduction of wild-type NF2 into Nf2ΔEx2 MSC reduced LIMK1 and LIMK2 levels. We show that pharmacological inhibition of LIMK with BMS-5 decreased the viability of Nf2ΔEx2 MSCs in a dose-dependent manner, but did not affect viability of control MSCs. Similarly, LIMK knockdown decreased viability of Nf2ΔEx2 MSCs. The decreased viability of Nf2ΔEx2 MSCs was not due to caspase-dependent or -independent apoptosis, but rather due to inhibition of cell cycle progression as evidenced by accumulation of cells in G2/M phase. Inhibition of LIMKs arrests cells in early mitosis by decreasing aurora A activation. Our results suggest that LIMKs are potential drug targets for NF2 and tumors associated with merlin deficiency.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Rouleau GA, Merel P, Lutchman M, Sanson M, Zucman J, Marineau C et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature 1993; 363: 515–521.
Asthagiri AR, Parry DM, Butman JA, Kim HJ, Tsilou ET, Zhuang Z et al. Neurofibromatosis type 2. Lancet 2009; 373: 1974–1986.
Baser ME, De Rienzo A, Altomare D, Balsara BR, Hedrick NM, Gutmann DH et al. Neurofibromatosis 2 and malignant mesothelioma. Neurology 2002; 59: 290–291.
Plotkin SR, Stemmer-Rachamimov AO, Barker FG 2nd, Halpin C, Padera TP, Tyrrell A et al. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med 2009; 361: 358–367.
Kalamarides M, Acosta MT, Babovic-Vuksanovic D, Carpen O, Cichowski K, Evans DG et al. Neurofibromatosis 2011: a report of the Children’s Tumor Foundation annual meeting. Acta Neuropathol 2012; 123: 369–380.
Li W, Cooper J, Karajannis MA, Giancotti FG . Merlin: a tumour suppressor with functions at the cell cortex and in the nucleus. EMBO Rep 2012; 13: 204–215.
Stamenkovic I, Yu Q . Merlin, a ‘magic’ linker between extracellular cues and intracellular signaling pathways that regulate cell motility, proliferation, and survival. Curr Protein Pept Sci 2010; 11: 471–484.
Pelton PD, Sherman LS, Rizvi TA, Marchionni MA, Wood P, Friedman RA et al. Ruffling membrane, stress fiber, cell spreading and proliferation abnormalities in human schwannoma cells. Oncogene 1998; 17: 2195–2209.
Kissil JL, Johnson KC, Eckman MS, Jacks T . Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J Biol Chem 2002; 277: 10394–10399.
Shaw RJ, Paez JG, Curto M, Yaktine A, Pruitt WM, Saotome I et al. The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev Cell 2001; 1: 63–72.
Xiao GH, Beeser A, Chernoff J, Testa JR . p21-Activated kinase links Rac/Cdc42 signaling to merlin. J Biol Chem 2002; 277: 883–886.
Kissil JL, Wilker EW, Johnson KC, Eckman MS, Yaffe MB, Jacks T . Merlin, the product of the Nf2 tumor suppressor gene, is an inhibitor of the p21-activated kinase, Pak1. Mol Cell 2003; 12: 841–849.
Nakai Y, Zheng Y, MacCollin M, Ratner N . Temporal control of Rac in Schwann cell-axon interaction is disrupted in NF2-mutant schwannoma cells. J Neurosci 2006; 26: 3390–3395.
Thaxton C, Lopera J, Bott M, Fernandez-Valle C . Neuregulin and laminin stimulate phosphorylation of the NF2 tumor suppressor in Schwann cells by distinct protein kinase A and p21-activated kinase-dependent pathways. Oncogene 2008; 27: 2705–2715.
Yi C, Wilker EW, Yaffe MB, Stemmer-Rachamimov A, Kissil JL . Validation of the p21-activated kinases as targets for inhibition in neurofibromatosis type 2. Cancer Res 2008; 68: 7932–7937.
Manetti F . LIM kinases are attractive targets with many macromolecular partners and only a few small molecule regulators. Med Res Rev 2011; 32: 968–998.
Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K et al. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature 1998; 393: 809–812.
Goyal P, Pandey D, Behring A, Siess W . Inhibition of nuclear import of LIMK2 in endothelial cells by protein kinase C-dependent phosphorylation at Ser-283. J Biol Chem 2005; 280: 27569–27577.
Scott RW, Olson MF . LIM kinases: function, regulation and association with human disease. J Mol Med (Berl) 2007; 85: 555–568.
Yokoo T, Toyoshima H, Miura M, Wang Y, Iida KT, Suzuki H et al. p57Kip2 regulates actin dynamics by binding and translocating LIM-kinase 1 to the nucleus. J Biol Chem 2003; 278: 52919–52923.
Amano T, Kaji N, Ohashi K, Mizuno K . Mitosis-specific activation of LIM motif-containing protein kinase and roles of cofilin phosphorylation and dephosphorylation in mitosis. J Biol Chem 2002; 277: 22093–22102.
Kaji N, Muramoto A, Mizuno K . LIM kinase-mediated cofilin phosphorylation during mitosis is required for precise spindle positioning. J Biol Chem 2008; 283: 4983–4992.
Sumi T, Hashigasako A, Matsumoto K, Nakamura T . Different activity regulation and subcellular localization of LIMK1 and LIMK2 during cell cycle transition. Exp Cell Res 2006; 312: 1021–1030.
Po’uha ST, Shum MS, Goebel A, Bernard O, Kavallaris M . LIM-kinase 2, a regulator of actin dynamics, is involved in mitotic spindle integrity and sensitivity to microtubule-destabilizing drugs. Oncogene 2010; 29: 597–607.
Nikonova AS, Astsaturov I, Serebriiskii IG, Dunbrack RL Jr., Golemis EA . Aurora A kinase (AURKA) in normal and pathological cell division. Cell Mol Life Sci 2013; 70: 661–687.
Barr AR, Gergely F . Aurora-A: the maker and breaker of spindle poles. J Cell Sci 2007; 120: 2987–2996.
Ritchey L, Ottman R, Roumanos M, Chakrabarti R . A functional cooperativity between Aurora A kinase and LIM kinase1: implication in the mitotic process. Cell Cycle 2012; 11: 296–309.
Johnson EO, Chang KH, Ghosh S, Venkatesh C, Giger K, Low PS et al. LIMK2 is a crucial regulator and effector of Aurora-A-kinase-mediated malignancy. J Cell Sci 2012; 125: 1204–1216.
Bagheri-Yarmand R, Mazumdar A, Sahin AA, Kumar R . LIM kinase 1 increases tumor metastasis of human breast cancer cells via regulation of the urokinase-type plasminogen activator system. Int J Cancer 2006; 118: 2703–2710.
Horita Y, Ohashi K, Mukai M, Inoue M, Mizuno K . Suppression of the invasive capacity of rat ascites hepatoma cells by knockdown of Slingshot or LIM kinase. J Biol Chem 2008; 283: 6013–6021.
Okamoto I, Pirker C, Bilban M, Berger W, Losert D, Marosi C et al. Seven novel and stable translocations associated with oncogenic gene expression in malignant melanoma. Neoplasia 2005; 7: 303–311.
McConnell BV, Koto K, Gutierrez-Hartmann A . Nuclear and cytoplasmic LIMK1 enhances human breast cancer progression. Mol Cancer 2011; 10: 75.
Manetti F . Recent findings confirm LIM domain kinases as emerging target candidates for cancer therapy. Curr Cancer Drug Targets 2012; 12: 543–560.
Giovannini M, Robanus-Maandag E, van der Valk M, Niwa-Kawakita M, Abramowski V, Goutebroze L et al. Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes Dev 2000; 14: 1617–1630.
Ross-Macdonald P, de Silva H, Guo Q, Xiao H, Hung CY, Penhallow B et al. Identification of a nonkinase target mediating cytotoxicity of novel kinase inhibitors. Mol Cancer Ther 2008; 11: 3490–3498.
Hung G, Li X, Faudoa R, Xeu Z, Kluwe L, Rhim JS et al. Establishment and characterization of a schwannoma cell line from a patient with neurofibromatosis 2. Int J Oncol 2002; 20: 475–482.
Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O et al. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 1998; 393: 805–809.
Bashour AM, Meng JJ, Ip W, MacCollin M, Ratner N . The neurofibromatosis type 2 gene product, merlin, reverses the F-actin cytoskeletal defects in primary human schwannoma cells. Mol Cell Biol 2002; 22: 1150–1157.
Sparrow N, Manetti ME, Bott M, Fabianac T, Petrilli A, Bates ML et al. The actin-severing protein cofilin is downstream of neuregulin signaling and is essential for Schwann cell myelination. J Neurosci 2012; 32: 5284–5297.
Cote MC, Lavoie JR, Houle F, Poirier A, Rousseau S, Huot J . Regulation of vascular endothelial growth factor-induced endothelial cell migration by LIM kinase 1-mediated phosphorylation of annexin 1. J Biol Chem 2010; 285: 8013–8021.
Thaxton C, Bott M, Walker B, Sparrow NA, Lambert S, Fernandez-Valle C . Schwannomin/merlin promotes Schwann cell elongation and influences myelin segment length. Mol Cell Neurosci 2011; 47: 1–9.
Iacovelli J, Lopera J, Bott M, Baldwin E, Khaled A, Uddin N et al. Serum and forskolin cooperate to promote G1 progression in Schwann cells by differentially regulating cyclin D1, cyclin E1, and p27Kip expression. Glia 2007; 55: 1638–1647.
Acknowledgements
We thank Dr Patrick Wood, The Miami Project to Cure Paralysis, Department of Neurological Surgery, University of Miami Miller School of Medicine, Miami, FL, USA, for vials of cultured human Schwann cells, Dr Maria Elisa Manetti for the Halo-tag NF2 plasmid, Nicklaus Sparrow and Marga Bott for their creation of the Nf2ΔEx2 MSCs and Rashell Hallford for animal husbandry. This work was supported in part by a DHHS/NIH award to CF-V (5R01DC10189), and AP is the recipient of a Young Investigator Award from the Children’s Tumor Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Petrilli, A., Copik, A., Posadas, M. et al. LIM domain kinases as potential therapeutic targets for neurofibromatosis type 2. Oncogene 33, 3571–3582 (2014). https://doi.org/10.1038/onc.2013.320
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.320
Keywords
This article is cited by
-
PLPP/CIN-mediated NF2 S10 dephosphorylation distinctly regulates kainate-induced seizure susceptibility and neuronal death through PAK1-NF-κB-COX-2-PTGES2 signaling pathway
Journal of Neuroinflammation (2023)
-
Cepharanthine sensitizes human triple negative breast cancer cells to chemotherapeutic agent epirubicin via inducing cofilin oxidation-mediated mitochondrial fission and apoptosis
Acta Pharmacologica Sinica (2022)
-
PLPP/CIN-mediated NF2-serine 10 dephosphorylation regulates F-actin stability and Mdm2 degradation in an activity-dependent manner
Cell Death & Disease (2021)
-
Requirement for LIM kinases in acute myeloid leukemia
Leukemia (2020)
-
LIMK1 and LIMK2 regulate cortical development through affecting neural progenitor cell proliferation and migration
Molecular Brain (2019)