Abstract
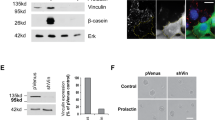
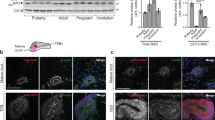
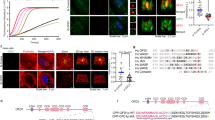
Actin filament-associated protein 1 (AFAP1) is an adaptor protein of cSrc that binds to filamentous actin and regulates the activity of this tyrosine kinase to affect changes to the organization of the actin cytoskeleton. In breast and prostate cancer cells, AFAP1 has been shown to regulate cellular responses requiring actin cytoskeletal changes such as adhesion, invadopodia formation and invasion. However, a normal physiologic role for AFAP1 has remained elusive. In this study, we generated an AFAP1 knockout mouse model that establishes a novel physiologic role for AFAP1 in lactation. Specifically, these animals displayed a defect in lactation that resulted in an inability to nurse efficiently. Histologically, the mammary glands of the lactating knockout mice were distinguished by the accumulation of large cytoplasmic lipid droplets in the alveolar epithelial cells. There was a reduction in lipid synthesis and the expression of lipogenic genes without a corresponding reduction in the production of β-casein, a milk protein. Furthermore, these defects were associated with histologic and biochemical signs of precocious involution. This study also demonstrated that AFAP1 responds to prolactin, a lactogenic hormone, by forming a complex with cSrc and becoming tyrosine phosphorylated. Taken together, these observations pointed to a defect in secretory activation. Certain characteristics of this phenotype mirrored the defect in secretory activation in the cSrc knockout mouse, but most importantly, the activity of cSrc in the mammary gland was reduced during early lactation in the AFAP1-null mouse and the localization of active cSrc at the apical surface of luminal epithelial cells during lactation was selectively lost in the absence of AFAP1. These data define, for the first time, the requirement of AFAP1 for the spatial and temporal regulation of cSrc activity in the normal breast, specifically for milk production.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Reynolds AB, Kanner SB, Bouton AH, Schaller MD, Weed SA, Flynn DC et al. SRChing for the substrates of Src. Oncogene (e-pub ahead of print 14 October 2013; doi:10.1038/onc.2013.416).
Flynn DC, Leu TH, Reynolds AB, Parsons JT . Identification and sequence analysis of cDNAs encoding a 110-kilodalton actin filament-associated pp60src substrate. Mol Cell Biol 1993; 13: 7892–7900.
Qian Y, Baisden JM, Cherezova L, Summy JM, Guappone-Koay A, Shi X et al. PC phosphorylation increases the ability of AFAP-110 to cross-link actin filaments. Mol Biol Cell 2002; 13: 2311–2322.
Guappone AC, Flynn DC . The integrity of the SH3 binding motif of AFAP-110 is required to facilitate tyrosine phosphorylation by, and stable complex formation with, Src. Mol Cell Biochem 1997; 175: 243–252.
Guappone AC, Weimer T, Flynn DC . Formation of a stable src-AFAP-110 complex through either an amino-terminal or a carboxy-terminal SH2-binding motif. Mol Carcinogen 1998; 22: 110–119.
Qian Y, Baisden JM, Zot HG, Van Winkle WB, Flynn DC . The carboxy terminus of AFAP-110 modulates direct interactions with actin filaments and regulates its ability to alter actin filament integrity and induce lamellipodia formation. Exp Cell Res 2000; 255: 102–113.
Dorfleutner A, Cho Y, Vincent D, Cunnick J, Lin H, Weed SA et al. Phosphorylation of AFAP-110 affects podosome lifespan in A7r5 cells. J Cell Sci 2008; 121 (Part 14): 2394–2405.
Gatesman A, Walker VG, Baisden JM, Weed SA, Flynn DC . Protein kinase Calpha activates c-Src and induces podosome formation via AFAP-110. Mol Cell Biol 2004; 24: 7578–7597.
Snyder BN, Cho Y, Qian Y, Coad JE, Flynn DC, Cunnick JM . AFAP1L1 is a novel adaptor protein of the AFAP family that interacts with cortactin and localizes to invadosomes. Eur J Cell Biol 2011; 90: 376–389.
Dorfleutner A, Stehlik C, Zhang J, Gallick GE, Flynn DC . AFAP-110 is required for actin stress fiber formation and cell adhesion in MDA-MB-231 breast cancer cells. J Cell Physiol 2007; 213: 740–749.
Baisden JM, Gatesman AS, Cherezova L, Jiang BH, Flynn DC . The intrinsic ability of AFAP-110 to alter actin filament integrity is linked with its ability to also activate cellular tyrosine kinases. Oncogene 2001; 20: 6607–6616.
Baisden JM, Qian Y, Zot HM, Flynn DC . The actin filament-associated protein AFAP-110 is an adaptor protein that modulates changes in actin filament integrity. Oncogene 2001; 20: 6435–6447.
Qian Y, Baisden JM, Westin EH, Guappone AC, Koay TC, Flynn DC . Src can regulate carboxy terminal interactions with AFAP-110, which influence self-association, cell localization and actin filament integrity. Oncogene 1998; 16: 2185–2195.
Zhang J, Park SI, Artime MC, Summy JM, Shah AN, Bomser JA et al. AFAP-110 is overexpressed in prostate cancer and contributes to tumorigenic growth by regulating focal contacts. J Clin Invest 2007; 117: 2962–2973.
Bourguignon LY, Wong G, Earle CA, Xia W . Interaction of low molecular weight hyaluronan with CD44 and toll-like receptors promotes the actin filament-associated protein 110-actin binding and MyD88-NFkappaB signaling leading to proinflammatory cytokine/chemokine production and breast tumor invasion. Cytoskeleton (Hoboken) 2011; 68: 671–693.
Parsons SJ, Parsons JT . Src family kinases, key regulators of signal transduction. Oncogene 2004; 23: 7906–7909.
Yeatman TJ . A renaissance for SRC. Nat Rev Cancer 2004; 4: 470–480.
Shimizu A, Maruyama T, Tamaki K, Uchida H, Asada H, Yoshimura Y . Impairment of decidualization in SRC-deficient mice. Biol Reprod 2005; 73: 1219–1227.
Kim H, Laing M, Muller W . c-Src-null mice exhibit defects in normal mammary gland development and ERalpha signaling. Oncogene 2005; 24: 5629–5636.
Watkin H, Richert MM, Lewis A, Terrell K, McManaman JP, Anderson SM . Lactation failure in Src knockout mice is due to impaired secretory activation. BMC. BMC Dev Biol 2008; 8: 6.
Marcotte R, Smith HW, Sanguin-Gendreau V, McDonough RV, Muller WJ . Mammary epithelial-specific disruption of c-Src impairs cell cycle progression and tumorigenesis. Proc Natl Acad Sci USA 2011; 109: 2808–2813.
Anderson SM, Rudolph MC, McManaman JL, Neville MC . Key stages in mammary gland development. Secretory activation in the mammary gland: it's not just about milk protein synthesis!. Breast Cancer Res 2007; 9: 204.
Naylor MJ, Oakes SR, Gardiner-Garden M, Harris J, Blazek K, Ho TW et al. Transcriptional changes underlying the secretory activation phase of mammary gland development. Mol Endocrinol 2005; 19: 1868–1883.
Marcotte R, Smith HW, Sanguin-Gendreau V, McDonough RV, Muller WJ . Mammary epithelial-specific disruption of c-Src impairs cell cycle progression and tumorigenesis. Proc Natl Acad Sci USA 2012; 109: 2808–2813.
Palmer CA, Neville MC, Anderson SM, McManaman JL . Analysis of lactation defects in transgenic mice. J Mammary Gland Biol Neoplasia 2006; 11: 269–282.
Richert MM, Schwertfeger KL, Ryder JW, Anderson SM . An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia 2000; 5: 227–241.
Acosta JJ, Munoz RM, Gonzalez L, Subtil-Rodriguez A, Dominguez-Caceres MA, Garcia-Martinez JM et al. Src mediates prolactin-dependent proliferation of T47D and MCF7 cells via the activation of focal adhesion kinase/Erk1/2 and phosphatidylinositol 3-kinase pathways. Mol Endocrinol 2003; 17: 2268–2282.
Berlanga JJ, Fresno Vara JA, Martin-Perez J, Garcia-Ruiz JP . Prolactin receptor is associated with c-src kinase in rat liver. Mol Endocrinol 1995; 9: 1461–1467.
Kabotyanski EB, Rosen JM . Signal transduction pathways regulated by prolactin and Src result in different conformations of activated Stat5b. J Biol Chem 2003; 278: 17218–17227.
Mirmohammadsadegh A, Hassan M, Bardenheuer W, Marini A, Gustrau A, Nambiar S et al. STAT5 phosphorylation in malignant melanoma is important for survival and is mediated through SRC and JAK1 kinases. J Invest Dermatol 2006; 126: 2272–2280.
Aksamitiene E, Achanta S, Kolch W, Kholodenko BN, Hoek JB, Kiyatkin A . Prolactin-stimulated activation of ERK1/2 mitogen-activated protein kinases is controlled by PI3-kinase/Rac/PAK signaling pathway in breast cancer cells. Cell Signal 2011; 23: 1794–1805.
Piazza TM, Lu JC, Carver KC, Schuler LA . SRC family kinases accelerate prolactin receptor internalization, modulating trafficking and signaling in breast cancer cells. Mol Endocrinol 2009; 23: 202–212.
Fresno Vara JA, Carretero MV, Geronimo H, Ballmer-Hofer K, Martin-Perez J . Stimulation of c-Src by prolactin is independent of Jak2. Biochem J 2000; 345 (Part 1): 17–24.
Garcia-Martinez JM, Calcabrini A, Gonzalez L, Martin-Forero E, Agullo-Ortuno MT, Simon V et al. A non-catalytic function of the Src family tyrosine kinases controls prolactin-induced Jak2 signaling. Cell Signal 2010; 22: 415–426.
Clump DA, Yu JJ, Cho Y, Gao R, Jett J, Zot H et al. A polymorphic variant of AFAP-110 enhances cSrc activity. Transl Oncol 2010; 3: 276–285.
Debnath J, Muthuswamy SK, Brugge JS . Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 2003; 30: 256–268.
Soriano P, Montgomery C, Geske R, Bradley A . Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 1991; 64: 693–702.
Hughes K, Wickenden JA, Allen JE, Watson CJ . Conditional deletion of Stat3 in mammary epithelium impairs the acute phase response and modulates immune cell numbers during post-lactational regression. J Pathol 2011; 227: 106–117.
Shackleford TJ, Zhang Q, Tian L, Vu TT, Korapati AL, Baumgartner AM et al. Stat3 and CCAAT/enhancer binding protein beta (C/EBP-beta) regulate Jab1/CSN5 expression in mammary carcinoma cells. Breast Cancer Res 2011; 13: R65.
Watson CJ, Kreuzaler PA . Remodeling mechanisms of the mammary gland during involution. Int J Dev Biol 2011; 55: 757–762.
Thangaraju M, Rudelius M, Bierie B, Raffeld M, Sharan S, Hennighausen L et al. C/EBPdelta is a crucial regulator of pro-apoptotic gene expression during mammary gland involution. Development 2005; 132: 4675–4685.
Linder S, Kopp P . Podosomes at a glance. J Cell Sci 2005; 118 (Part 10): 2079–2082.
Sandilands E, Brunton VG, Frame MC . The membrane targeting and spatial activation of Src, Yes and Fyn is influenced by palmitoylation and distinct RhoB/RhoD endosome requirements. J Cell Sci 2007; 120 (Part 15): 2555–2564.
Sandilands E, Cans C, Fincham VJ, Brunton VG, Mellor H, Prendergast GC et al. RhoB and actin polymerization coordinate Src activation with endosome-mediated delivery to the membrane. Dev Cell 2004; 7: 855–869.
Scott KE, Wheeler FB, Davis AL, Thomas MJ, Ntambi JM, Seals DF et al. Metabolic regulation of invadopodia and invasion by acetyl-CoA carboxylase 1 and de novo lipogenesis. PLoS One 2012; 7: e29761.
Liu Y, Chen BP, Lu M, Zhu Y, Stemerman MB, Chien S et al. Shear stress activation of SREBP1 in endothelial cells is mediated by integrins. Arterioscl Thromb Vasc Biol 2002; 22: 76–81.
McManaman JL, Palmer CA, Anderson S, Schwertfeger K, Neville MC . Regulation of milk lipid formation and secretion in the mouse mammary gland. Adv Exp Med Biol 2004; 554: 263–279.
Ogg SL, Weldon AK, Dobbie L, Smith AJ, Mather IH . Expression of butyrophilin (Btn1a1) in lactating mammary gland is essential for the regulated secretion of milk-lipid droplets. Proc Natl Acad Sci USA 2004; 101: 10084–10089.
Vorbach C, Scriven A, Capecchi MR . The housekeeping gene xanthine oxidoreductase is necessary for milk fat droplet enveloping and secretion: gene sharing in the lactating mammary gland. Genes Dev 2002; 16: 3223–3235.
Franke WW, Heid HW, Grund C, Winter S, Freudenstein C, Schmid E et al. Antibodies to the major insoluble milk fat globule membrane-associated protein: specific location in apical regions of lactating epithelial cells. J Cell Biol 1981; 89: 485–494.
Amato PA, Loizzi RF . The identification and localization of actin and actin-like filaments in lactating guinea pig mammary gland alveolar cells. Cell Motil 1981; 1: 329–347.
Dimri M, Naramura M, Duan L, Chen J, Ortega-Cava C, Chen G et al. Modeling breast cancer-associated c-Src and EGFR overexpression in human MECs: c-Src and EGFR cooperatively promote aberrant three-dimensional acinar structure and invasive behavior. Cancer rRes 2007; 67: 4164–4172.
Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R et al. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol 2005; 171: 717–728.
Abramoff MD, Magalhaes PJ, Ram SJ . Image Processing with ImageJ. Biophoton Int 2004; 11: 36–42.
Acknowledgements
This work is supported by NIH Grant R01CA060731-15A1. We thank Victor Malloy for his care of the mice and Wafa Atamna for cell sorting. We also thank Raj Kumar and John Arnott for the careful reading of our manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Cunnick, J., Kim, S., Hadsell, J. et al. Actin filament-associated protein 1 is required for cSrc activity and secretory activation in the lactating mammary gland. Oncogene 34, 2640–2649 (2015). https://doi.org/10.1038/onc.2014.205
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.205
This article is cited by
-
Up-regulated lncRNA AFAP1-AS1 indicates a poor prognosis and promotes carcinogenesis of breast cancer
Breast Cancer (2019)
-
Long non-coding RNA AFAP1-AS1 plays an oncogenic role in promoting cell migration in non-small cell lung cancer
Cellular and Molecular Life Sciences (2018)
-
Whole exome sequencing in three families segregating a pediatric case of sarcoidosis
BMC Medical Genomics (2018)