Abstract
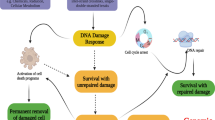
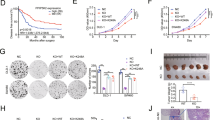
The DNA damage response (DDR) helps to maintain genome integrity, suppress tumorigenesis and mediate the radiotherapeutic and chemotherapeutic effects on cancer. Here we report that p57Kip2, a cyclin-dependent kinase (CDK) inhibitor implicated in the development of tumor-prone Beckwith–Wiedemann syndrome, is an effector molecule of the DNA-damage response. Genotoxic stress induces p57Kip2 expression via the bone morphogenetic protein-Smad1 and Atm-p38MAPK-Atf2 pathways in p53-proficient or -deficient cells and requires the Smad1-Atf2 complex that facilitates their recruitment to the p57Kip2 promoter. Elevated p57Kip2 induces G1/S phase cell cycle arrest but inhibits cell death in response to DNA damage and acts in parallel with p53 to suppress cell transformation and tumor formation. p57Kip2 is also upregulated in stage I and II clinical rectal tumor samples, likely due to genome instability of precancerous and/or early cancer cells. Targeting p57Kip2 in primary rectal cancer cells and tumor models resulted in increased sensitivity to doxorubicin, suggesting that p57Kip2 has a role in chemoresistance, which is consistent with its pro-survival function. These findings place p57Kip2 in DDR and uncover molecular mechanisms by which p57Kip2 suppresses tumorigenesis and causes chemoresistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Lord CJ, Ashworth A . The DNA damage response and cancer therapy. Nature 2012; 481: 287–294.
Jackson SP, Bartek J . The DNA-damage response in human biology and disease. Nature 2009; 461: 1071–1078.
Ciccia A, Elledge SJ . The DNA damage response: making it safe to play with knives. Mol Cell 2010; 40: 179–204.
Belyi VA, Ak P, Markert E, Wang H, Hu W, Puzio-Kuter A et al. The origins and evolution of the p53 family of genes. Cold Spring Harb Perspect Biol 2010; 2: a001198.
Cheok CF, Verma CS, Baselga J, Lane DP . Translating p53 into the clinic. Nat Rev Clin Oncol 2011; 8: 25–37.
Muller PA, Vousden KH . p53 mutations in cancer. Nat Cell Biol 2013; 15: 2–8.
Vogelstein B, Lane D, Levine AJ . Surfing the p53 network. Nature 2000; 408: 307–310.
Chau JF, Jia D, Wang Z, Liu Z, Hu Y, Zhang X et al. A crucial role for bone morphogenetic protein-Smad1 signalling in the DNA damage response. Nat Commun 2012; 3: 836.
Lai KP, Leong WF, Chau JF, Jia D, Zeng L, Liu H et al. S6K1 is a multifaceted regulator of Mdm2 that connects nutrient status and DNA damage response. EMBO J 2010; 29: 2994–3006.
Reinhardt HC, Yaffe MB . Kinases that control the cell cycle in response to DNA damage: Chk1, Chk2, and MK2. Curr Opin Cell Biol 2009; 21: 245–255.
Starostina NG, Kipreos ET . Multiple degradation pathways regulate versatile CIP/KIP CDK inhibitors. Trends Cell Biol 2012; 22: 33–41.
Besson A, Dowdy SF, Roberts JM . CDK inhibitors: cell cycle regulators and beyond. Dev Cell 2008; 14: 159–169.
Zhang P, Liégeois NJ, Wong C, Finegold M, Hou H, Thompson JC et al. Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature 1997; 387: 151–158.
Zhang P, Wong C, Liu D, Finegold M, Harper JW, Elledge SJ et al. p21(CIP1) and p57(KIP2) control muscle differentiation at the myogenin step. Genes Dev 1999; 13: 213–222.
Susaki E, Nakayama K, Yamasaki L, Nakayama KI . Common and specific roles of the related CDK inhibitors p27 and p57 revealed by a knock-in mouse model. Proc Natl Acad Sci USA 2009; 106: 5192–5197.
Susaki E, Nakayama KI . Functional similarities and uniqueness of p27 and p57: insight from a knock-in mouse model. Cell Cycle 2009; 8: 2497–2501.
Pateras IS, Apostolopoulou K, Niforou K, Kotsinas A, Gorgoulis VG . p57KIP2: "Kip"ing the cell under control. Mol Cancer Res 2009; 7: 1902–1919.
Deng C, Zhang P, Harper JW, Elledge SJ, Leder P . Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 1995; 82: 675–684.
Mairet-Coello G, Tury A, Van Buskirk E, Robinson K, Genestine M, DiCicco-Bloom E . p57(KIP2) regulates radial glia and intermediate precursor cell cycle dynamics and lower layer neurogenesis in developing cerebral cortex. Development 2012; 139: 475–487.
Ullah Z, Kohn MJ, Yagi R, Vassilev LT, DePamphilis ML . Differentiation of trophoblast stem cells into giant cells is triggered by p57/Kip2 inhibition of CDK1 activity. Genes Dev 2008; 22: 3024–3036.
Matsumoto A, Takeishi S, Kanie T, Susaki E, Onoyama I, Tateishi Y et al. p57 is required for quiescence and maintenance of adult hematopoietic stem cells. Cell Stem Cell 2011; 9: 262–271.
Furutachi S, Matsumoto A, Nakayama KI, Gotoh Y . p57 controls adult neural stem cell quiescence and modulates the pace of lifelong neurogenesis. EMBO J 2011; 32: 970–981.
Borriello A, Caldarelli I, Bencivenga D, Criscuolo M, Cucciolla V, Tramontano A et al. p57(Kip2) and cancer: time for a critical appraisal. Mol Cancer Res 2011; 9: 1269–1284.
Kavanagh E, Joseph B . The hallmarks of CDKN1C (p57, KIP2) in cancer. Biochim Biophys Acta 2011; 1816: 50–56.
Zhao R, Yang HY, Shin J, Phan L, Fang L, Che TF et al. CDK inhibitor p57 (Kip2) is downregulated by Akt during HER2-mediated tumorigenicity. Cell Cycle 2013; 12: 935–943.
Yang X, Karuturi RK, Sun F, Aau M, Yu K, Shao R et al. CDKN1C (p57) is a direct target of EZH2 and suppressed by multiple epigenetic mechanisms in breast cancer cells. PLoS One 2009; 4: e5011.
Pateras IS, Apostolopoulou K, Koutsami M, Evangelou K, Tsantoulis P, Liloglou T et al. Downregulation of the KIP family members p27(KIP1) and p57(KIP2) by SKP2 and the role of methylation in p57(KIP2) inactivation in nonsmall cell lung cancer. Int J Cancer Suppl 2006; 119: 2546–2556.
Caspary T, Cleary MA, Perlman EJ, Zhang P, Elledge SJ, Tilghman SM . Oppositely imprinted genes p57(Kip2) and igf2 interact in a mouse model for Beckwith-Wiedemann syndrome. Genes Dev 1999; 13: 3115–3124.
Matsuoka S, Edwards MC, Bai C, Parker S, Zhang P, Baldini A et al. p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev 1995; 9: 650–662.
Zhang X, Jia D, Liu H, Zhu N, Zhang W, Feng J et al. Identification of 5-Iodotubercidin as a genotoxic drug with anti-cancer potential. PLoS One 2013; 8: e62527.
Massague J, Seoane J, Wotton D . Smad transcription factors. Genes Dev 2005; 19: 2783–2810.
Gosselet FP, Magnaldo T, Culerrier RM, Sarasin A, Ehrhart JC . BMP2 and BMP6 control p57(Kip2) expression and cell growth arrest/terminal differentiation in normal primary human epidermal keratinocytes. Cell Signal 2007; 19: 731–739.
Urano T, Yashiroda H, Muraoka M, Tanaka K, Hosoi T, Inoue S et al. p57(Kip2) is degraded through the proteasome in osteoblasts stimulated to proliferation by transforming growth factor beta1. J Biol Chem 1999; 274: 12197–12200.
Schmierer B, Hill CS . TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol 2007; 8: 970–982.
ten Dijke P, Hill CS . New insights into TGF-beta-Smad signalling. Trends Biochem Sci 2004; 29: 265–273.
Balint E, Phillips AC, Kozlov S, Stewart CL, Vousden KH . Induction of p57(KIP2) expression by p73beta. Proc Natl Acad Sci USA 2002; 99: 3529–3534.
Giovannini C, Gramantieri L, Minguzzi M, Fornari F, Chieco P, Grazi GL et al. CDKN1C/P57 is regulated by the Notch target gene Hes1 and induces senescence in human hepatocellular carcinoma. Am J Pathol 2012; 181: 413–422.
Cannell IG, Kong YW, Johnston SJ, Chen ML, Collins HM, Dobbyn HC et al. p38 MAPK/MK2-mediated induction of miR-34c following DNA damage prevents Myc-dependent DNA replication. Proc Natl Acad Sci USA 2010; 107: 5375–5380.
Reinhardt HC, Hasskamp P, Schmedding I, Morandell S, van Vugt MA, Wang X et al. DNA damage activates a spatially distinct late cytoplasmic cell-cycle checkpoint network controlled by MK2-mediated RNA stabilization. Mol Cell 2010; 40: 34–49.
Yang Y, Xia F, Hermance N, Mabb A, Simonson S, Morrissey S et al. A cytosolic ATM/NEMO/RIP1 complex recruits TAK1 to mediate the NF-kappaB and p38 mitogen-activated protein kinase (MAPK)/MAPK-activated protein 2 responses to DNA damage. Mol Cell Biol 2011; 31: 2774–2786.
Wang QE, Han C, Zhao R, Wani G, Zhu Q, Gong L et al. p38 MAPK- and Akt-mediated p300 phosphorylation regulates its degradation to facilitate nucleotide excision repair. Nucleic Acids Res 2012; 41: 1722–1733.
Hopker K, Hagmann H, Khurshid S, Chen S, Hasskamp P, Seeger-Nukpezah T et al. AATF/Che-1 acts as a phosphorylation-dependent molecular modulator to repress p53-driven apoptosis. EMBO J 2012; 31: 3961–3975.
Barascu A, Le Chalony C, Pennarun G, Genet D, Imam N, Lopez B et al. Oxidative stress induces an ATM-independent senescence pathway through p38 MAPK-mediated lamin B1 accumulation. EMBO J 2012; 31: 1080–1094.
Raman M, Earnest S, Zhang K, Zhao Y, Cobb MH . TAO kinases mediate activation of p38 in response to DNA damage. EMBO J 2007; 26: 2005–2014.
Gozdecka M, Breitwieser W . The roles of ATF2 (activating transcription factor 2) in tumorigenesis. Biochem Soc Trans 2012; 40: 230–234.
Bhoumik A, Takahashi S, Breitweiser W, Shiloh Y, Jones N, Ronai Z . ATM-dependent phosphorylation of ATF2 is required for the DNA damage response. Mol Cell 2005; 18: 577–587.
Bhoumik A, Singha N, O'Connell MJ, Ronai ZA . Regulation of TIP60 by ATF2 modulates ATM activation. J Biol Chem 2008; 283: 17605–17614.
Li S, Ezhevsky S, Dewing A, Cato MH, Scortegagna M, Bhoumik A et al. Radiation sensitivity and tumor susceptibility in ATM phospho-mutant ATF2 mice. Genes Cancer 2010; 1: 316–330.
Gartel AL . p21(WAF1/CIP1) and cancer: a shifting paradigm? BioFactors 2009; 35: 161–164.
Cazzalini O, Scovassi AI, Savio M, Stivala LA, Prosperi E . Multiple roles of the cell cycle inhibitor p21(CDKN1A) in the DNA damage response. Mutat Res 2010; 704: 12–20.
Bartek J, Bartkova J, Lukas J . DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene 2007; 26: 7773–7779.
Gu J, Chen N . Current status of rectal cancer treatment in China. Colorectal Dis 2013; 07: 677–2012.
Velimezi G, Liontos M, Vougas K, Roumeliotis T, Bartkova J, Sideridou M et al. Functional interplay between the DNA-damage-response kinase ATM and ARF tumour suppressor protein in human cancer. Nat Cell Biol 2013; 15: 967–977.
Borriello A, Cucciolla V, Oliva A, Zappia V, Della Ragione F . p27Kip1 metabolism: a fascinating labyrinth. Cell Cycle 2007; 6: 1053–1061.
Sugihara E, Kanai M, Saito S, Nitta T, Toyoshima H, Nakayama K et al. Suppression of centrosome amplification after DNA damage depends on p27 accumulation. Cancer Res 2006; 66: 4020–4029.
Joaquin M, Gubern A, González-Nuñez D, Josué Ruiz E, Ferreiro I, de Nadal E et al. The p57 CDKi integrates stress signals into cell-cycle progression to promote cell survival upon stress. EMBO J 2012; 31: 2952–2964.
Kavanagh E, Vlachos P, Emourgeon V, Rodhe J, Joseph B . p57(KIP2) control of actin cytoskeleton dynamics is responsible for its mitochondrial pro-apoptotic effect. Cell Death Dis 2012; 3: e311.
Vlachos P, Nyman U, Hajji N, Joseph B . The cell cycle inhibitor p57(Kip2) promotes cell death via the mitochondrial apoptotic pathway. Cell Death Differ 2007; 14: 1497–1507.
Reinhardt HC, Aslanian AS, Lees JA, Yaffe MB . p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage. Cancer Cell 2007; 11: 175–189.
Reimold AM, Grusby MJ, Kosaras B, Fries JW, Mori R, Maniwa S et al. Chondrodysplasia and neurological abnormalities in ATF-2-deficient mice. Nature 1996; 379: 262–265.
Tremblay KD, Dunn NR, Robertson EJ . Mouse embryos lacking Smad1 signals display defects in extra-embryonic tissues and germ cell formation. Development 2001; 128: 3609–3621.
Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB et al. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev 2005; 19: 1175–1187.
Xie M, Zhang D, Dyck JR, Li Y, Zhang H, Morishima M et al. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc Natl Acad Sci USA 2006; 103: 17378–17383.
Acknowledgements
We thank Lina Gao, IH In, C Deyu, GC Hong, L Soh, J Lin and T Cheng for technical assistance and Boehringer Ingelheim, Novartis, Dr Michael D. Schneider, Dr Ye-Guang Chen, Dr Yibin Wang, Dr Nelson J Dusetti, Dr Olivier Delattre, Dr Philipp Kaldis and Dr Michael Kastan for providing constructs, reagents and mice. The work was supported by grants from the Ministry of Science and Technology of China (The National Key Scientific Program (2012CB966901 to BL) and the National Natural Science Foundation of China (81130039, 31071229 and 81121001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Jia, H., Cong, Q., Chua, J. et al. p57Kip2 is an unrecognized DNA damage response effector molecule that functions in tumor suppression and chemoresistance. Oncogene 34, 3568–3581 (2015). https://doi.org/10.1038/onc.2014.287
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.287
This article is cited by
-
TCF4 (E2-2) harbors tumor suppressive functions in SHH medulloblastoma
Acta Neuropathologica (2019)
-
The LIM protein AJUBA promotes colorectal cancer cell survival through suppression of JAK1/STAT1/IFIT2 network
Oncogene (2017)
-
The dynamic behavior of Ect2 in response to DNA damage
Scientific Reports (2016)