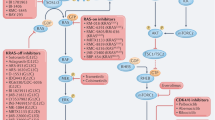
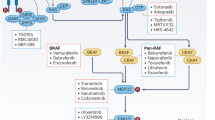
Abstract
The RAS-RAF-MEK1/2-ERK1/2 pathway is a key signal transduction pathway in the cells. Critically, it remains constitutively active in approximately 30% of human cancers, having key roles in cancer development, maintenance and progression, while being responsible for poorer prognosis and drug resistance. Consequently, the inhibition of this pathway has been the subject of intense research for >25 years. The advent of better patient screening techniques has increasingly shown that upstream regulators like RAS and RAF remain persistently mutated in many cancer types. These gain-of-function mutations, such as KRAS-4BG12V/G13D/Q61K, NRASQ61L/Q61R or BRAFV600E, lead to tremendous increase in their activities, resulting in constitutively active extracellular signal–regulated kinase 1/2 (ERK1/2). They were not efficiently targeted by the first-generation inhibitors such as Lonafarnib or Sorafenib, which were essentially broad spectrum inhibitors targeting pan-RAS and pan-RAF, respectively. This triggered the development of the second-generation inhibitors selective against the mutated proteins. Second generation inhibitors such as Vemurafenib (Zelboraf) and Dabrafenib (Tafinlar) targeting BRAFV600E, Trametinib (Mekinist) targeting MEK1/2 and the first generation pan-RAF inhibitor Sorafenib (Nexavar) have already been approved for treating renal, hepatocellular, thyroid cancers and BRAFV600E/K harboring metastatic melanoma. Others against RAF and MEK1/2 are presently undergoing clinical trials. Their success would depend on the better understanding of the acquired resistance mechanisms to these drugs in the cancer cells and the identification of predictive biomarkers for the proper administration of suitable inhibitor(s).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Geest CR, Coffer PJ . MAPK signaling pathways in the regulation of hematopoiesis. J Leukoc Biol 2009; 86: 237–250.
Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 2001; 22: 153–183.
Wada T, Penninger JM . Mitogen-activated protein kinases in apoptosis regulation. Oncogene 2004; 23: 2838–2849.
Songyang Z, Lu KP, Kwon YT, Tsai LH, Filhol O, Cochet C et al. A structural basis for substrate specificities of protein Ser/Thr kinases: primary sequence preference of casein kinases I and II, NIMA, phosphorylase kinase, calmodulin-dependent kinase II, CDK5, and Erk1. Mol Cell Biol 1996; 16: 6486–6493.
Roux PP, Blenis J . ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 2004; 68: 320–344.
Cargnello M, Roux PP . Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev 2011; 75: 50–83.
Zhao Y, Adjei AA . The clinical development of MEK inhibitors. Nat Rev Clin Oncol 2014; 11: 385–400.
Fisher R, Larkin J . Vemurafenib: a new treatment for BRAF-V600 mutated advanced melanoma. Cancer Manage Res 2012; 4: 243–252.
Mandal R, Raab M, Matthess Y, Becker S, Knecht R, Strebhardt K . pERK 1/2 inhibit Caspase-8 induced apoptosis in cancer cells by phosphorylating it in a cell cycle specific manner. Mol Oncol 2014; 8: 232–249.
Rudd CE . MAPK p38: alternative and nonstressful in T cells. Nat Immunol 2005; 6: 368–370.
Adjei AA . Blocking oncogenic Ras signaling for cancer therapy. J Natl Cancer Inst 2001; 93: 1062–1074.
Poulikakos PI, Rosen N . Mutant BRAF melanomas—dependence and resistance. Cancer Cell 2011; 19: 11–15.
Roberts PJ, Der CJ . Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007; 26: 3291–3310.
Descot A, Hoffmann R, Shaposhnikov D, Reschke M, Ullrich A, Posern G . Negative regulation of the EGFR-MAPK cascade by actin-MAL-mediated Mig6/Errfi-1 induction. Mol Cell 2009; 35: 291–304.
Liu X, Yan S, Zhou T, Terada Y, Erikson RL . The MAP kinase pathway is required for entry into mitosis and cell survival. Oncogene 2004; 23: 763–776.
Dai B, Zhao XF, Mazan-Mamczarz K, Hagner P, Corl S, Bahassi EM et al. Functional and molecular interactions between ERK and CHK2 in diffuse large B-cell lymphoma. Nat Commun 2011; 2: 402.
Friday BB, Adjei AA . Advances in targeting the Ras/Raf/MEK/Erk mitogen-activated protein kinase cascade with MEK inhibitors for cancer therapy. Clin Cancer Res 2008; 14: 342–346.
Schneider H, Wang H, Raab M, Valk E, Smith X, Lovatt M et al. Adaptor SKAP-55 binds p21 activating exchange factor RasGRP1 and negatively regulates the p21-ERK pathway in T-cells. PLoS One 2008; 3: e1718.
Pucci B, Indelicato M, Paradisi V, Reali V, Pellegrini L, Aventaggiato M et al. ERK-1 MAP kinase prevents TNF-induced apoptosis through bad phosphorylation and inhibition of Bax translocation in HeLa Cells. J Cell Biochem 2009; 108: 1166–1174.
Little AS, Smith PD, Cook SJ . Mechanisms of acquired resistance to ERK1/2 pathway inhibitors. Oncogene 2013; 32: 1207–1215.
Schafer B, Gschwind A, Ullrich A . Multiple G-protein-coupled receptor signals converge on the epidermal growth factor receptor to promote migration and invasion. Oncogene 2004; 23: 991–999.
Gollob JA, Wilhelm S, Carter C, Kelley SL . Role of Raf kinase in cancer: therapeutic potential of targeting the Raf/MEK/ERK signal transduction pathway. Semin Oncol 2006; 33: 392–406.
Steinmetz R, Wagoner HA, Zeng P, Hammond JR, Hannon TS, Meyers JL et al. Mechanisms regulating the constitutive activation of the extracellular signal-regulated kinase (ERK) signaling pathway in ovarian cancer and the effect of ribonucleic acid interference for ERK1/2 on cancer cell proliferation. Mol Endocrinol 2004; 18: 2570–2582.
Lo RS . Receptor tyrosine kinases in cancer escape from BRAF inhibitors. Cell Res 2012; 22: 945–947.
Satyamoorthy K, Li G, Gerrero MR, Brose MS, Volpe P, Weber BL et al. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res 2003; 63: 756–759.
Towatari M, Iida H, Tanimoto M, Iwata H, Hamaguchi M, Saito H . Constitutive activation of mitogen-activated protein kinase pathway in acute leukemia cells. Leukemia 1997; 11: 479–484.
Bollag G, Tsai J, Zhang J, Zhang C, Ibrahim P, Nolop K et al. Vemurafenib: the first drug approved for BRAF-mutant cancer. Nat Rev Drug Discov 2012; 11: 873–886.
Huang T, Karsy M, Zhuge J, Zhong M, Liu D . B-Raf and the inhibitors: from bench to bedside. J Hematol Oncol 2013; 6: 30.
Fernandez-Medarde A, Santos E . Ras in cancer and developmental diseases. Genes Cancer 2011; 2: 344–358.
McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 2007; 1773: 1263–1284.
Holderfield M, Deuker MM, McCormick F, McMahon M, Targeting RAF . kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat Rev Cancer 2014; 14: 455–467.
Menzies AM, Long GV . Dabrafenib and trametinib, alone and in combination for BRAF-mutant metastatic melanoma. Clin Cancer Res 2014; 20: 2035–2043.
Corcoran RB, Settleman J, Engelman JA . Potential therapeutic strategies to overcome acquired resistance to BRAF or MEK inhibitors in BRAF mutant cancers. Oncotarget 2011; 2: 336–346.
Martinelli E, Troiani T, D'Aiuto E, Morgillo F, Vitagliano D, Capasso A et al. Antitumor activity of pimasertib, a selective MEK 1/2 inhibitor, in combination with PI3K/mTOR inhibitors or with multi-targeted kinase inhibitors in pimasertib-resistant human lung and colorectal cancer cells. Int J Cancer 2013; 133: 2089–2101.
Cantwell-Dorris ER, O'Leary JJ, Sheils OM . BRAFV600E: implications for carcinogenesis and molecular therapy. Mol Cancer Ther 2011; 10: 385–394.
Menzies AM, Long GV, Murali R . Dabrafenib and its potential for the treatment of metastatic melanoma. Drug Des Dev Ther 2012; 6: 391–405.
Ascierto PA, Schadendorf D, Berking C, Agarwala SS, van Herpen CM, Queirolo P et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol 2013; 14: 249–256.
Hoeflich KP, Herter S, Tien J, Wong L, Berry L, Chan J et al. Antitumor efficacy of the novel RAF inhibitor GDC-0879 is predicted by BRAFV600E mutational status and sustained extracellular signal-regulated kinase/mitogen-activated protein kinase pathway suppression. Cancer Res 2009; 69: 3042–3051.
Sanada M, Hidaka M, Takagi Y, Takano TY, Nakatsu Y, Tsuzuki T et al. Modes of actions of two types of anti-neoplastic drugs, dacarbazine and ACNU, to induce apoptosis. Carcinogenesis 2007; 28: 2657–2663.
Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004; 64: 7099–7109.
Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov 2006; 5: 835–844.
Luke JJ, Hodi FS . Vemurafenib and BRAF inhibition: a new class of treatment for metastatic melanoma. Clin Cancer Res 2012; 18: 9–14.
Yang H, Higgins B, Kolinsky K, Packman K, Go Z, Iyer R et al. RG7204 (PLX4032), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models. Cancer Res 2010; 70: 5518–5527.
Johansson CH, Brage SE . BRAF inhibitors in cancer therapy. Pharmacol Ther 2014; 142: 176–182.
Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci USA 2008; 105: 3041–3046.
Stuart DD, Li N, Poon DJ, Aardalen K, Kaufman S, Merritt H et al. Abstract 3790: preclinical profile of LGX818: A potent and selective RAF kinase inhibitor. Cancer Res 2012; 72 (Suppl 8): 3790.
Dickson MA, Gordon MS, Edelman G, Bendell JC, Kudchadkar RR, LoRusso PM et al. Phase I study of XL281 (BMS-908662), a potent oral RAF kinase inhibitor, in patients with advanced solid tumors. Invest New Drugs 2015; 33: 349–356.
Schwartz GK, Robertson S, Shen A, Wang E, Pace L, Dials H et al. A phase I study of XL281, a selective oral RAF kinase inhibitor, in patients (Pts) with advanced solid tumors. ASCO Meeting Abstr 2009; 27 (Suppl 15): 3513.
Su Y, Vilgelm AE, Kelley MC, Hawkins OE, Liu Y, Boyd KL et al. RAF265 inhibits the growth of advanced human melanoma tumors. Clin Cancer Res 2012; 18: 2184–2198.
Stuart D, Aardalen K, Venetsanakos E, Nagel T, Wallroth M, Batt D et al RAF265 is a potent Raf kinase inhibitor with selective anti-proliferative activity in vitro and in vivo. AACR Meeting Abstr 2008: 4876.
Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 2010; 468: 968–972.
Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol 2011; 29: 3085–3096.
Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 2012; 487: 500–504.
Villanueva J, Vultur A, Herlyn M . Resistance to BRAF inhibitors: unraveling mechanisms and future treatment options. Cancer Res 2011; 71: 7137–7140.
Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N . RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 2010; 464: 427–430.
Cox AD, Der CJ . The raf inhibitor paradox: unexpected consequences of targeted drugs. Cancer Cell 2010; 17: 221–223.
Hall-Jackson CA, Eyers PA, Cohen P, Goedert M, Boyle FT, Hewitt N et al. Paradoxical activation of Raf by a novel Raf inhibitor. Chem Biol 1999; 6: 559–568.
Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature 2010; 464: 431–435.
Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 2010; 140: 209–221.
Sourvinos G, Tsatsanis C, Spandidos DA . Overexpression of the Tpl-2/Cot oncogene in human breast cancer. Oncogene 1999; 18: 4968–4973.
Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, Moriceau G et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature 2011; 480: 387–390.
Trunzer K, Pavlick AC, Schuchter L, Gonzalez R, McArthur GA, Hutson TE et al. Pharmacodynamic effects and mechanisms of resistance to vemurafenib in patients with metastatic melanoma. J Clin Oncol 2013; 31: 1767–1774.
Rizos H, Menzies AM, Pupo GM, Carlino MS, Fung C, Hyman J et al. BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res 2014; 20: 1965–1977.
Emery CM, Vijayendran KG, Zipser MC, Sawyer AM, Niu L, Kim JJ et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci USA 2009; 106: 20411–20416.
Hatzivassiliou G, Haling JR, Chen H, Song K, Price S, Heald R et al. Mechanism of MEK inhibition determines efficacy in mutant KRAS- versus BRAF-driven cancers. Nature 2013; 501: 232–236.
Little AS, Balmanno K, Sale MJ, Smith PD, Cook SJ . Tumour cell responses to MEK1/2 inhibitors: acquired resistance and pathway remodelling. Biochem Soc Trans 2012; 40: 73–78.
Wang D, Boerner SA, Winkler JD, LoRusso PM . Clinical experience of MEK inhibitors in cancer therapy. Biochim Biophys Acta 2007; 1773: 1248–1255.
Fremin C, Meloche S . From basic research to clinical development of MEK1/2 inhibitors for cancer therapy. J Hematol Oncol 2010; 3: 8.
Davies BR, Logie A, McKay JS, Martin P, Steele S, Jenkins R et al. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol Cancer Ther 2007; 6: 2209–2219.
Adjei AA, Cohen RB, Franklin W, Morris C, Wilson D, Molina JR et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol 2008; 26: 2139–2146.
Chang-Yew Leow C, Gerondakis S, Spencer A . MEK inhibitors as a chemotherapeutic intervention in multiple myeloma. Blood Cancer J 2013; 3: e105.
Kirkwood JM, Bastholt L, Robert C, Sosman J, Larkin J, Hersey P et al. Phase II, open-label, randomized trial of the MEK1/2 inhibitor selumetinib as monotherapy versus temozolomide in patients with advanced melanoma. Clin Cancer Res 2012; 18: 555–567.
Akinleye A, Furqan M, Mukhi N, Ravella P, Liu D . MEK and the inhibitors: from bench to bedside. J Hematol Oncol 2013; 6: 27.
Honda K, Yamamoto N, Nokihara H, Tamura Y, Asahina H, Yamada Y et al. Phase I and pharmacokinetic/pharmacodynamic study of RO5126766, a first-in-class dual Raf/MEK inhibitor, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 2013; 72: 577–584.
Martinez-Garcia M, Banerji U, Albanell J, Bahleda R, Dolly S, Kraeber-Bodere F et al. First-in-human, phase I dose-escalation study of the safety, pharmacokinetics, and pharmacodynamics of RO5126766, a first-in-class dual MEK/RAF inhibitor in patients with solid tumors. Clin Cancer Res 2012; 18: 4806–4819.
Sale MJ, Cook SJ . Intrinsic and acquired resistance to MEK1/2 inhibitors in cancer. Biochem Soc Trans 2014; 42: 776–783.
Long GV, Stroyakovsky DL, Gogas H, Levchenko E, de Braud F, Larkin JMG et al. COMBI-d: A randomized, double-blinded, Phase III study comparing the combination of dabrafenib and trametinib to dabrafenib and trametinib placebo as first-line therapy in patients (pts) with unresectable or metastatic BRAFV600E/Kmutation-positive cutaneous melanoma. ASCO Meeting Abstr 2014; 32 (Suppl 15): 9011.
Girotti MR, Lopes F, Preece N, Niculescu-Duvaz D, Zambon A, Davies L et al. Paradox-breaking RAF inhibitors that also target SRC are effective in drug-resistant BRAF mutant melanoma. Cancer Cell 2015; 27: 85–96.
Ramos JW . The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int J Biochem Cell Biol 2008; 40: 2707–2719.
Eblen ST, Slack-Davis JK, Tarcsafalvi A, Parsons JT, Weber MJ, Catling AD . Mitogen-activated protein kinase feedback phosphorylation regulates MEK1 complex formation and activation during cellular adhesion. Mol Cell Biol 2004; 24: 2308–2317.
Dougherty MK, Muller J, Ritt DA, Zhou M, Zhou XZ, Copeland TD et al. Regulation of Raf-1 by direct feedback phosphorylation. Mol Cell 2005; 17: 215–224.
Ritt DA, Monson DM, Specht SI, Morrison DK . Impact of feedback phosphorylation and Raf heterodimerization on normal and mutant B-Raf signaling. Mol Cell Biol 2010; 30: 806–819.
Lavoie H, Therrien M . Regulation of RAF protein kinases in ERK signalling. Nat Rev Mol Cell Biol 2015; 16: 281–298.
Corbalan-Garcia S, Yang SS, Degenhardt KR, Bar-Sagi D . Identification of the mitogen-activated protein kinase phosphorylation sites on human Sos1 that regulate interaction with Grb2. Mol Cell Biol 1996; 16: 5674–5682.
Lito P, Saborowski A, Yue J, Solomon M, Joseph E, Gadal S et al. Disruption of CRAF-mediated MEK activation is required for effective MEK inhibition in KRAS mutant tumors. Cancer Cell 2014; 25: 697–710.
Gilmartin AG, Bleam MR, Groy A, Moss KG, Minthorn EA, Kulkarni SG et al. GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition. Clin Cancer Res 2011; 17: 989–1000.
Narita Y, Okamoto K, Kawada MI, Takase K, Minoshima Y, Kodama K et al. Novel ATP-competitive MEK inhibitor E6201 is effective against vemurafenib-resistant melanoma harboring the MEK1-C121S mutation in a preclinical model. Mol Cancer Ther 2014; 13: 823–832.
Villanueva J, Infante JR, Krepler C, Reyes-Uribe P, Samanta M, Chen HY et al. Concurrent MEK2 mutation and BRAF amplification confer resistance to BRAF and MEK inhibitors in melanoma. Cell Rep 2013; 4: 1090–1099.
Corcoran RB, Dias-Santagata D, Bergethon K, Iafrate AJ, Settleman J, Engelman JA . BRAF gene amplification can promote acquired resistance to MEK inhibitors in cancer cells harboring the BRAF V600E mutation. Sci Signal 2010; 3: ra84.
Little AS, Balmanno K, Sale MJ, Newman S, Dry JR, Hampson M et al. Amplification of the driving oncogene, KRAS or BRAF, underpins acquired resistance to MEK1/2 inhibitors in colorectal cancer cells. Sci Signal 2011; 4: ra17.
Hocker HJ, Cho KJ, Chen CY, Rambahal N, Sagineedu SR, Shaari K et al. Andrographolide derivatives inhibit guanine nucleotide exchange and abrogate oncogenic Ras function. Proc Natl Acad Sci USA 2013; 110: 10201–10206.
Baker NM, Der CJ . Cancer: drug for an 'undruggable' protein. Nature 2013; 497: 577–578.
Zimmermann G, Papke B, Ismail S, Vartak N, Chandra A, Hoffmann M et al. Small molecule inhibition of the KRAS-PDEdelta interaction impairs oncogenic KRAS signalling. Nature 2013; 497: 638–642.
Maurer T, Garrenton LS, Oh A, Pitts K, Anderson DJ, Skelton NJ et al. Small-molecule ligands bind to a distinct pocket in Ras and inhibit SOS-mediated nucleotide exchange activity. Proc Natl Acad Sci USA 2012; 109: 5299–5304.
Xiao Z, Li L, Li Y, Zhou W, Cheng J, Liu F et al. Rasfonin, a novel 2-pyrone derivative, induces ras-mutated Panc-1 pancreatic tumor cell death in nude mice. Cell Death Dis 2014; 5: e1241.
Khazak V, Astsaturov I, Serebriiskii IG, Golemis EA . Selective Raf inhibition in cancer therapy. Expert Opin Ther Targets 2007; 11: 1587–1609.
Strebhardt K, Ullrich A . Paul Ehrlich's magic bullet concept: 100 years of progress. Nat Rev Cancer 2008; 8: 473–480.
Yeh JJ, Routh ED, Rubinas T, Peacock J, Martin TD, Shen XJ et al. KRAS/BRAF mutation status and ERK1/2 activation as biomarkers for MEK1/2 inhibitor therapy in colorectal cancer. Mol Cancer Ther 2009; 8: 834–843.
Tentler JJ, Nallapareddy S, Tan AC, Spreafico A, Pitts TM, Morelli MP et al. Identification of predictive markers of response to the MEK1/2 inhibitor selumetinib (AZD6244) in K-ras-mutated colorectal cancer. Mol Cancer Ther 2010; 9: 3351–3362.
Jing J, Greshock J, Holbrook JD, Gilmartin A, Zhang X, McNeil E et al. Comprehensive predictive biomarker analysis for MEK inhibitor GSK1120212. Mol Cancer Ther 2012; 11: 720–729.
Lam ET, Ringel MD, Kloos RT, Prior TW, Knopp MV, Liang J et al. Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. J Clin Oncol 2010; 28: 2323–2330.
Hong DS, Camacho L, Ng C, Wright J, Newman RA, Moulder S et al. Phase I study of tipifarnib and sorafenib in patients with biopsiable advanced cancers (NCI protocol 7156 supported by NCI grant UO1 CA062461). ASCO Meeting Abstr 2007; 25 (Suppl 18): 3549.
Chintala L, Kurzrock R, Fu S, Naing A, Wheler JJ, Moulder SL et al. Phase I study of tipifarnib and sorafenib in patients with biopsiable advanced cancer (NCI protocol 7156). ASCO Meeting Abstr 2008; 26 (Suppl 15): 3593.
Gollob JA, Rathmell WK, Richmond TM, Marino CB, Miller EK, Grigson G et al. Phase II trial of sorafenib plus interferon alfa-2b as first- or second-line therapy in patients with metastatic renal cell cancer. J Clin Oncol 2007; 25: 3288–3295.
Infante JR, Falchook GS, Lawrence DP, Weber JS, Kefford RF, Bendell JC et al. Phase I/II study to assess safety, pharmacokinetics, and efficacy of the oral MEK 1/2 inhibitor GSK1120212 (GSK212) dosed in combination with the oral BRAF inhibitor GSK2118436 (GSK436). ASCO Meeting Abstr 2011; 29 (Suppl 15): CRA8503.
Azijli K, Stelloo E, Peters GJ, VDE AJ . New developments in the treatment of metastatic melanoma: immune checkpoint inhibitors and targeted therapies. Anticancer Res 2014; 34: 1493–1505.
Caponigro G, Cao ZA, Zhang X, Wang HQ, Fritsch CM, Stuart DD . Abstract 2337: efficacy of the RAF/PI3K{alpha}/anti-EGFR triple combination LGX818 + BYL719 + cetuximab in BRAFV600E colorectal tumor models. Cancer Res 2013; 73 (Suppl 8): 2337.
Ascierto PA, De Maio E, Bertuzzi S, Palmieri G, Halaban R, Hendrix M et al. Future perspectives in melanoma research. Meeting report from the "Melanoma Research: a bridge Naples-USA. Naples, December 6th-7th 2010". J Transl Med 2011; 9: 32.
Lim HY, Yen C-J, Tak W-Y, Heo J, Choi HJ, Lin C-Y et al. A phase II trial of MEK inhibitor BAY 86-9766 in combination with sorafenib as first-line systemic treatment for patients with unresectable hepatocellular carcinoma (HCC). ASCO Meeting Abstr 2012; 30 (Suppl 15): 4103.
Juric D, Soria J-C, Sharma S, Banerji U, Azaro A, Desai J et al. A phase 1b dose-escalation study of BYL719 plus binimetinib (MEK162) in patients with selected advanced solid tumors. ASCO Meeting Abstr 2014; 32 (Suppl 15): 9051.
Finn RS, Javle MM, Tan BR, Weekes CD, Bendell JC, Patnaik A et al. A phase I study of MEK inhibitor MEK162 (ARRY-438162) in patients with biliary tract cancer. ASCO Meeting Abstr 2012; 30 (Suppl 4): 220.
Riess H, Van Laethem J-L, Martens UM, Heinemann V, Michl P, Peeters M et al. Phase II study of the MEK inhibitor refametinib (BAY 86-9766) in combination with gemcitabine in patients with unresectable, locally advanced, or metastatic pancreatic cancer: biomarker results. ASCO Meeting Abstr 2014; 32 (Suppl 15): 4129.
Van Laethem J-L, Jassem J, Heinemann V, Weekes CD, Bridgewater JA, Cascinu S et al. Phase II study of refametinib (BAY 86-9766), an allosteric dual MEK 1/2 inhibitor, and gemcitabine in patients with unresectable, locally advanced, or metastatic pancreatic cancer. ASCO Meeting Abstr 2014; 32 (Suppl 15): 4025.
Adjei AA, LoRusso P, Ribas A, Sosman JA, Pavlick AC, Dy GK et al. Phase I, dose-escalation study of the investigational drug TAK-733, an oral MEK inhibitor, in patients (pts) with advanced solid tumors. ASCO Meeting Abstr 2013; 31 (Suppl 15): 2528.
Fabrey R, O'Connell S, Stanton A, Chakravarty A, Gangolli E, Ecsedy J et al. Abstract 3739: TAK-733, an investigational, selective MEK1/2 inhibitor, in combination with alisertib (MLN8237), an investigational, selective Aurora A kinase inhibitor is tolerated and results in additive to synergistic antitumor activity: results from in vivo studies. Cancer Res 2012; 72 (Suppl 8): 3739.
Collins S, Blair D, Zarycki J, Szynal C, Gangolli E, Vincent P et al. Abstract 3738: A rationale for combining the targeted investigational agents TAK-733, a MEK1/2 inhibitor, with alisertib (MLN8237), an Aurora A kinase inhibitor, for cancer therapy. Cancer Res 2012; 72 (Suppl 8): 3738.
Leijen S, Middleton MR, Tresca P, Kraeber-Bodere F, Dieras V, Scheulen ME et al. Phase I dose-escalation study of the safety, pharmacokinetics, and pharmacodynamics of the MEK inhibitor RO4987655 (CH4987655) in patients with advanced solid tumors. Clin Cancer Res 2012; 18: 4794–4805.
Britten C, Wainberg Z, Tabernero J, Alsina Maqueda M, Leong S, Sessa C et al. 358 A multi-arm phase 1 dose escalation study of safety, pharmacokinetics, and pharmacodynamics of the dual PI3K/mTOR inhibitors PF-04691502 (oral) and PF-05212384 (IV) in combination with the MEK inhibitor PD-0325901 or Irinotecan in patients with advanced cancer. Eur J Cancer 2012; 48 (Suppl 6): 109.
Cohen RB, Aamdal S, Nyakas M, Cavallin M, Green D, Learoyd M et al. A phase I dose-finding, safety and tolerability study of AZD8330 in patients with advanced malignancies. Eur J Cancer 2013; 49: 1521–1529.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mandal, R., Becker, S. & Strebhardt, K. Stamping out RAF and MEK1/2 to inhibit the ERK1/2 pathway: an emerging threat to anticancer therapy. Oncogene 35, 2547–2561 (2016). https://doi.org/10.1038/onc.2015.329
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.329
This article is cited by
-
Overexpression of CYP11A1 recovers cell cycle distribution in renal cell carcinoma Caki-1
Cancer Cell International (2022)
-
Clinical Pharmacokinetics and Pharmacodynamics of Selumetinib
Clinical Pharmacokinetics (2021)
-
Overview of current targeted therapy in gallbladder cancer
Signal Transduction and Targeted Therapy (2020)
-
HSP90/AXL/eIF4E-regulated unfolded protein response as an acquired vulnerability in drug-resistant KRAS-mutant lung cancer
Oncogenesis (2019)
-
Post-treatment de-phosphorylation of p53 correlates with dasatinib responsiveness in malignant melanoma
BMC Cell Biology (2018)