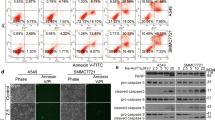
Abstract
Based on the central role of the ubiquitin–proteasome system (UPS) in the degradation of cellular proteins, proteasome inhibition has been considered an attractive approach for anticancer therapy. Deubiquitinases (DUBs) remove ubiquitin conjugates from diverse substrates; therefore, they are essential regulators of the UPS. DUB inhibitors, especially the inhibitors of proteasomal DUBs are becoming a research hotspot in targeted cancer therapy. Previous studies have shown that metal complexes, such as copper and zinc complexes, can induce cancer cell apoptosis through inhibiting UPS function. Moreover, we have found that copper pyrithione inhibits both 19S proteasome-associated DUBs and 20S proteasome activity with a mechanism distinct from that of the classical 20S proteasome inhibitor bortezomib. In the present study, we reveal that (i) nickel pyrithione complex (NiPT) potently inhibits the UPS via targeting the 19S proteasome-associated DUBs (UCHL5 and USP14), without effecting on the 20S proteasome; (ii) NiPT selectively induces proteasome inhibition and apoptosis in cultured tumor cells and cancer cells from acute myeloid leukemia human patients; and (iii) NiPT inhibits proteasome function and tumor growth in nude mice. This study, for the first time, uncovers a nickel complex as an effective inhibitor of the 19S proteasomal DUBs and suggests a potentially new strategy for cancer treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Adams J . The proteasome: structure, function, and role in the cell. Cancer Treat Rev 2003; 29: 3–9.
Mani A, Gelmann EP . The ubiquitin-proteasome pathway and its role in cancer. J Clin Oncol 2005; 23: 4776–4789.
Burger AM, Seth AK . The ubiquitin-mediated protein degradation pathway in cancer: therapeutic implications. Eur J Cancer 2004; 40: 2217–2229.
Paramore A, Frantz S . Bortezomib. Nat Rev Drug Discov 2003; 2: 611–612.
Chen D, Frezza M, Schmitt S, Kanwar J, Dou QP . Bortezomib as the first proteasome inhibitor anticancer drug: current status and future perspectives. Curr Cancer Drug Targets 2011; 11: 239–253.
Adams J . The development of proteasome inhibitors as anticancer drugs. Cancer Cell 2004; 5: 417–421.
Fraile JM, Quesada V, Rodriguez D, Freije JM, Lopez-Otin C . Deubiquitinases in cancer: new functions and therapeutic options. Oncogene 2012; 31: 2373–2388.
Singhal S, Taylor MC, Baker RT . Deubiquitylating enzymes and disease. BMC Biochem 2008; 9: S3.
Sacco JJ, Coulson JM, Clague MJ, Urbe S . Emerging roles of deubiquitinases in cancer-associated pathways. IUBMB Life 2010; 62: 140–157.
Todi SV, Paulson HL . Balancing act: deubiquitinating enzymes in the nervous system. Trends Neurosci 2011; 34: 370–382.
Verma R, Aravind L, Oania R, McDonald WH, Yates JR 3rd, Koonin EV et al. Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science 2002; 298: 611–615.
Koulich E, Li X, DeMartino GN . Relative structural and functional roles of multiple deubiquitylating proteins associated with mammalian 26S proteasome. Mol Biol Cell 2008; 19: 1072–1082.
Yao T, Song L, Xu W, DeMartino GN, Florens L, Swanson SK et al. Proteasome recruitment and activation of the Uch37 deubiquitinating enzyme by Adrm1. Nat Cell Biol 2006; 8: 994–1002.
D'Arcy P, Brnjic S, Olofsson MH, Fryknas M, Lindsten K, De Cesare M et al. Inhibition of proteasome deubiquitinating activity as a new cancer therapy. Nat Med 2011; 17: 1636–1640.
Ventii KH, Wilkinson KD . Protein partners of deubiquitinating enzymes. Biochem J 2008; 414: 161–175.
Jamieson ER, Lippard SJ . Structure, recognition, and processing of cisplatin-DNA adducts. Chem Rev 1999; 99: 2467–2498.
Pabla N, Huang S, Mi QS, Daniel R, Dong Z . ATR-Chk2 signaling in p53 activation and DNA damage response during cisplatin-induced apoptosis. J Biol Chem 2008; 283: 6572–6583.
Roos WP, Kaina B . DNA damage-induced cell death by apoptosis. Trends Mol Med 2006; 12: 440–450.
Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM . A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol 2000; 10: 886–895.
Milacic V, Chen D, Giovagnini L, Diez A, Fregona D, Dou QP . Pyrrolidine dithiocarbamate-zinc(II) and -copper(II) complexes induce apoptosis in tumor cells by inhibiting the proteasomal activity. Toxicol Appl Pharmacol 2008; 231: 24–33.
Liu N, Liu C, Li X, Liao S, Song W, Yang C et al. A novel proteasome inhibitor suppresses tumor growth via targeting both 19S proteasome deubiquitinases and 20S proteolytic peptidases. Sci Rep 2014; 4: 5240.
Verani CN . Metal complexes as inhibitors of the 26S proteasome in tumor cells. J Inorg Biochem 2012; 106: 59–67.
Tailler M, Senovilla L, Lainey E, Thepot S, Metivier D, Sebert M et al. Antineoplastic activity of ouabain and pyrithione zinc in acute myeloid leukemia. Oncogene 2012; 31: 3536–3546.
Bence NF, Sampat RM, Kopito RR . Impairment of the ubiquitin-proteasome system by protein aggregation. Science 2001; 292: 1552–1555.
Liu J, Chen Q, Huang W, Horak KM, Zheng H, Mestril R et al. Impairment of the ubiquitin-proteasome system in desminopathy mouse hearts. FASEB J 2006; 20: 362–364.
Cvek B, Milacic V, Taraba J, Dou QP . Ni(II), Cu(II), and Zn(II) diethyldithiocarbamate complexes show various activities against the proteasome in breast cancer cells. J Med Chem 2008; 51: 6256–6258.
Frezza M, Hindo SS, Tomco D, Allard MM, Cui QC, Heeg MJ et al. Comparative activities of nickel(II) and zinc(II) complexes of asymmetric [NN'O] ligands as 26S proteasome inhibitors. Inorg Chem 2009; 48: 5928–5937.
Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A . H2AX: the histone guardian of the genome. DNA Repair (Amst) 2004; 3: 959–967.
Denkhaus E, Salnikow K . Nickel essentiality, toxicity, and carcinogenicity. Crit Rev Oncol Hematol 2002; 42: 35–56.
Chen J, Huang YW, Liu G, Afrasiabi Z, Sinn E, Padhye S et al. The cytotoxicity and mechanisms of 1,2-naphthoquinone thiosemicarbazone and its metal derivatives against MCF-7 human breast cancer cells. Toxicol Appl Pharmacol 2004; 197: 40–48.
Atasever B, Ulkuseven B, Bal-Demirci T, Erdem-Kuruca S, Solakoglu Z . Cytotoxic activities of new iron(III) and nickel(II) chelates of some S-methyl-thiosemicarbazones on K562 and ECV304 cells. Invest New Drugs 2010; 28: 421–432.
Tan J, Zhu L, Wang B . From GC-rich DNA binding to the repression of survivin gene for quercetin nickel (II) complex: implications for cancer therapy. Biometals 2010; 23: 1075–1084.
Florea AM, Busselberg D . Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 2011; 3: 1351–1371.
Chen D, Frezza M, Shakya R, Cui QC, Milacic V, Verani CN et al. Inhibition of the proteasome activity by gallium(III) complexes contributes to their anti prostate tumor effects. Cancer Res 2007; 67: 9258–9265.
Liu N, Li X, Huang H, Zhao C, Liao S, Yang C et al. Clinically used antirheumatic agent auranofin is a proteasomal deubiquitinase inhibitor and inhibits tumor growth. Oncotarget 2014; 5: 5453–5471.
Dinning AJ, Al-Adham IS, Austin P, Charlton M, Collier PJ . Pyrithione biocide interactions with bacterial phospholipid head groups. J Appl Microbiol 1998; 85: 132–140.
Tian Z, D'Arcy P, Wang X, Ray A, Tai YT, Hu Y et al. A novel small molecule inhibitor of deubiquitylating enzyme USP14 and UCHL5 induces apoptosis in multiple myeloma and overcomes bortezomib resistance. Blood 2014; 123: 706–716.
Li X, Liu S, Huang H, Liu N, Zhao C, Liao S et al. Gambogic acid is a tissue-specific proteasome inhibitor in vitro and in vivo. Cell Rep 2013; 3: 211–222.
Chen X, Shi X, Zhao C, Li X, Lan X, Liu S et al. Anti-rheumatic agent auranofin induced apoptosis in chronic myeloid leukemia cells resistant to imatinib through both Bcr/Abl-dependent and -independent mechanisms. Oncotarget 2014; 5: 9118–9132.
Huang H, Zhang X, Li S, Liu N, Lian W, McDowell E et al. Physiological levels of ATP negatively regulate proteasome function. Cell Res 2010; 20: 1372–1385.
Shi X, Lan X, Chen X, Zhao C, Li X, Liu S et al. Gambogic acid induces apoptosis in diffuse large B-cell lymphoma cells via inducing proteasome inhibition. Sci Rep 2015; 5: 9694.
Huang H, Liu N, Guo H, Liao S, Li X, Yang C et al. L-carnitine is an endogenous HDAC inhibitor selectively inhibiting cancer cell growth in vivo and in vitro. PLoS One 2012; 7: e49062.
Yang H, Zhou P, Huang H, Chen D, Ma N, Cui Q et al. Shikonin exerts antitumor activity via proteasome inhibition and cell death induction in vitro and in vivo. Int J Cancer 2009; 124: 2450–2459.
Dayal S, Sparks A, Jacob J, Allende-Vega N, Lane DP, Saville MK . Suppression of the deubiquitinating enzyme USP5 causes the accumulation of unanchored polyubiquitin and the activation of p53. J Biol Chem 2009; 284: 5030–5041.
Shi X, Chen X, Li X, Lan X, Zhao C, Liu S et al. Gambogic acid induces apoptosis in imatinib-resistant chronic myeloid leukemia cells via inducing proteasome inhibition and caspase-dependent Bcr-Abl downregulation. Clin Cancer Res 2014; 20: 151–163.
Acknowledgements
We thank Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Sun Yat-Sen Memorial Hospital and Sun Yat-Sen University for flow cytometry detection. This work was supported by the National High Technology Research and Development Program of China (2006AA02Z4B5), NSFC (81272451/H1609, 81472762/H1609), MOE (20134423110002) and Guangdong Key Laboratory of Urology (2010A060801016) (to JL) and in part by US NIH grants HL072166 and HL085629 (to XW).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Zhao, C., Chen, X., Zang, D. et al. A novel nickel complex works as a proteasomal deubiquitinase inhibitor for cancer therapy. Oncogene 35, 5916–5927 (2016). https://doi.org/10.1038/onc.2016.114
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.114
This article is cited by
-
The AUTOTAC chemical biology platform for targeted protein degradation via the autophagy-lysosome system
Nature Communications (2022)
-
Deubiquitinases in hematological malignancies
Biomarker Research (2021)
-
Regulation of Bax-dependent apoptosis by mitochondrial deubiquitinase USP30
Cell Death Discovery (2021)
-
Pyrithione metal (Cu, Ni, Ru) complexes as photo-catalysts for styrene oxide production
Scientific Reports (2021)
-
The deubiquitylase UCHL3 maintains cancer stem-like properties by stabilizing the aryl hydrocarbon receptor
Signal Transduction and Targeted Therapy (2020)