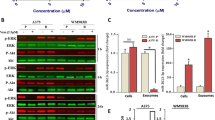
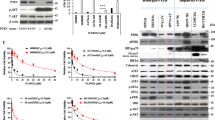
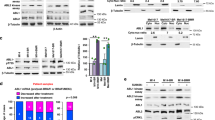
Abstract
Activating BRAF mutations promote constitutive activation of the mitogen-activated protein kinase (MAPK) signaling pathway and are common in a variety of human malignancies, including melanoma and colon cancer. Several small molecule BRAF inhibitors such as vemurafenib have been developed and demonstrate remarkable clinical efficacy. However, resistance typically emerges in most melanoma patients. Studies have demonstrated that reactivation of MAPK signaling via CRAF overexpression and dysregulation is a mechanism for vemurafenib resistance in melanoma. Prohibitins (PHBs) are highly conserved proteins that are thought to control the cell cycle, senescence and tumor suppression. PHB1 is essential for CRAF-mediated ERK1/2 activation through direct binding to CRAF. We developed a CRAF-mediated model of vemurafenib resistance in melanoma cells to assess the importance of the interaction between CRAF and PHB1 in resistance to BRAF-targeting agents. We demonstrate that CRAF overexpression renders melanoma cells resistant to BRAF-targeting agents. Moreover, treatment with the natural compound rocaglamide A disrupts the interaction between PHB and CRAF in melanoma cells, thus reducing MEK1/2 and ERK1/2 signaling, inhibiting melanoma cell growth and inducing apoptosis. The efficacy of these compounds was also demonstrated in a human melanoma xenograft model. Taken together, these data suggest that PHB1 may serve as a novel, druggable target in CRAF-mediated vemurafenib resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hertzman Johansson C, Egyhazi Brage S . BRAF inhibitors in cancer therapy. Pharmacol Ther 2014; 142: 176–182.
Barras D . BRAF mutation in colorectal cancer: an update. BiomarkCancer 2015; 7: 9–12.
Li DD, Zhang YF, Xu HX, Zhang XP . The role of BRAF in the pathogenesis of thyroid carcinoma. Front Biosci (Landmark Ed) 2015; 20: 1068–1078.
Martin-Liberal J, Larkin J . Vemurafenib for the treatment of BRAF mutant metastatic melanoma. Future Oncol 2015; 11: 579–589.
Nguyen-Ngoc T, Bouchaab H, Adjei AA, Peters S . BRAF alterations as therapeutic targets in non-small-cell lung cancer. J Thor Oncol 2015; 10: 1396–1403.
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S et al. Mutations of the BRAF gene in human cancer. Nature 2002; 417: 949–954.
Tan YH, Liu Y, Eu KW, Ang PW, Li WQ, Salto-Tellez M et al. Detection of BRAF V600E mutation by pyrosequencing. Pathology 2008; 40: 295–298.
Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 2004; 116: 855–867.
Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 2012; 366: 707–714.
Spagnolo F, Ghiorzo P, Orgiano L, Pastorino L, Picasso V, Tornari E et al. BRAF-mutant melanoma: treatment approaches, resistance mechanisms, and diagnostic strategies. Onco Targets Ther 2015; 8: 157–168.
Mao M, Tian F, Mariadason JM, Tsao CC, Lemos R Jr, Dayyani F et al. Resistance to BRAF inhibition in BRAF-mutant colon cancer can be overcome with PI3K inhibition or demethylating agents. Clin Cancer Res 2013; 19: 657–667.
Zhou TB, Qin YH . Signaling pathways of prohibitin and its role in diseases. J Recept Signal Transduct Res 2013; 33: 28–36.
Peng YT, Chen P, Ouyang RY, Song L . Multifaceted role of prohibitin in cell survival and apoptosis. Apoptosis 2015; 20: 1135–1149.
Asamoto M, Cohen SM . Prohibitin gene is overexpressed but not mutated in rat bladder carcinomas and cell lines. Cancer Lett 1994; 83: 201–207.
Chowdhury I, Garcia-Barrio M, Harp D, Thomas K, Matthews R, Thompson WE . The emerging roles of prohibitins in folliculogenesis. Front Biosci (Elite Ed) 2012; 4: 690–699.
Sripathi SR, He W, Atkinson CL, Smith JJ, Liu Z, Elledge BM et al. Mitochondrial-nuclear communication by prohibitin shuttling under oxidative stress. Biochemistry 2011; 50: 8342–8351.
Osman C, Haag M, Potting C, Rodenfels J, Dip PV, Wieland FT et al. The genetic interactome of prohibitins: coordinated control of cardiolipin and phosphatidylethanolamine by conserved regulators in mitochondria. J Cell Biol 2009; 184: 583–596.
Rajalingam K, Wunder C, Brinkmann V, Churin Y, Hekman M, Sievers C et al. Prohibitin is required for Ras-induced Raf-MEK-ERK activation and epithelial cell migration. Nat Cell Biol 2005; 7: 837–843.
Sullivan RJ, Flaherty K . MAP kinase signaling and inhibition in melanoma. Oncogene 2013; 32: 2373–2379.
Rajalingam K, Schreck R, Rapp UR, Albert S . Ras oncogenes and their downstream targets. Biochim Biophys Acta 2007; 1773: 1177–1195.
Zhu JY, Lavrik IN, Mahlknecht U, Giaisi M, Proksch P, Krammer PH et al. The traditional Chinese herbal compound rocaglamide preferentially induces apoptosis in leukemia cells by modulation of mitogen-activated protein kinase activities. Int J Cancer 2007; 121: 1839–1846.
Polier G, Neumann J, Thuaud F, Ribeiro N, Gelhaus C, Schmidt H et al. The natural anticancer compounds rocaglamides inhibit the Raf-MEK-ERK pathway by targeting prohibitin 1 and 2. Chem Biol 2012; 19: 1093–1104.
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364: 2507–2516.
Paraiso KH, Xiang Y, Rebecca VW, Abel EV, Chen YA, Munko AC et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res 2011; 71: 2750–2760.
Smalley KS, Lioni M, Dalla Palma M, Xiao M, Desai B, Egyhazi S et al. Increased cyclin D1 expression can mediate BRAF inhibitor resistance in BRAF V600E-mutated melanomas. Mol Cancer Ther 2008; 7: 2876–2883.
Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature 2012; 487: 505–509.
Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 2010; 467: 596–599.
Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 2010; 468: 973–977.
Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1 R/PI3K. Cancer Cell 2010; 18: 683–695.
Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol 2011; 29: 3085–3096.
Emery CM, Vijayendran KG, Zipser MC, Sawyer AM, Niu L, Kim JJ et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci USA 2009; 106: 20411–20416.
Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 2010; 468: 968–972.
Choi J, Landrette SF, Wang T, Evans P, Bacchiocchi A, Bjornson R et al. Identification of PLX4032-resistance mechanisms and implications for novel RAF inhibitors. Pigment Cell Melanoma Res 2014; 27: 253–262.
Su F, Bradley WD, Wang Q, Yang H, Xu L, Higgins B et al. Resistance to selective BRAF inhibition can be mediated by modest upstream pathway activation. Cancer Res 2012; 72: 969–978.
Al-Obeidi FA, Lam KS . Development of inhibitors for protein tyrosine kinases. Oncogene 2000; 19: 5690–5701.
Luan Z, He Y, Alattar M, Chen Z, He F . Targeting the prohibitin scaffold-CRAF kinase interaction in RAS-ERK-driven pancreatic ductal adenocarcinoma. Mol Cancer 2014; 13: 38.
Hossen MN, Kajimoto K, Akita H, Hyodo M, Ishitsuka T, Harashima H . Ligand-based targeted delivery of a peptide modified nanocarrier to endothelial cells in adipose tissue. J Control Release 2010; 147: 261–268.
Jakob JA, Bassett RL Jr, Ng CS, Curry JL, Joseph RW, Alvarado GC et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer 2012; 118: 4014–4023.
Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 2010; 140: 209–221.
Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N . RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 2010; 464: 427–430.
Robert C, Arnault JP, Mateus C . RAF inhibition and induction of cutaneous squamous cell carcinoma. Curr Opin Oncol 2011; 23: 177–182.
Katona TM, Jones TD, Wang M, Eble JN, Billings SD, Cheng L . Genetically heterogeneous and clonally unrelated metastases may arise in patients with cutaneous melanoma. Am J Surg Pathol 2007; 31: 1029–1037.
Yancovitz M, Litterman A, Yoon J, Ng E, Shapiro RL, Berman RS et al. Intra- and inter-tumor heterogeneity of BRAF(V600E))mutations in primary and metastatic melanoma. PLoS One 2012; 7: e29336.
Ross JA, Nagy ZS, Kirken RA . The PHB1/2 phosphocomplex is required for mitochondrial homeostasis and survival of human T cells. J Biol Chem 2008; 283: 4699–4713.
Doudican N, Rodriguez A, Osman I, Orlow SJ . Mebendazole induces apoptosis via Bcl-2 inactivation in chemoresistant melanoma cells. Mol Cancer Res 2008; 6: 1308–1315.
Acknowledgements
We thank the NYUMC Immunohistochemistry Core for their help with the immunohistochemistry experiments. This study was supported by the Orbuch & Brand Pilot Grant Program for Cancers of the Skin. The NYUMC Immunohistochemistry Core performed the immunohistochemistry experiments outlined in this manuscript. This shared resource is partially supported by the Cancer Center Support Grant, P30CA016087, at the Laura and Isaac Perlmutter Cancer Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Doudican, N., Orlow, S. Inhibition of the CRAF/prohibitin interaction reverses CRAF-dependent resistance to vemurafenib. Oncogene 36, 423–428 (2017). https://doi.org/10.1038/onc.2016.214
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.214
This article is cited by
-
Targeting CRAF kinase in anti-cancer therapy: progress and opportunities
Molecular Cancer (2023)
-
Targeted intervention of eIF4A1 inhibits EMT and metastasis of pancreatic cancer cells via c-MYC/miR-9 signaling
Cancer Cell International (2021)
-
Mechanisms of Resistance to BRAF-Targeted Melanoma Therapies
American Journal of Clinical Dermatology (2021)
-
Prohibitin ligands: a growing armamentarium to tackle cancers, osteoporosis, inflammatory, cardiac and neurological diseases
Cellular and Molecular Life Sciences (2020)
-
Significance of prohibitin domain family in tumorigenesis and its implication in cancer diagnosis and treatment
Cell Death & Disease (2018)