Abstract
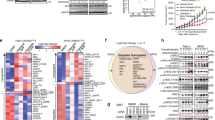
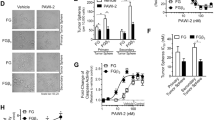
Pancreatic ductal adenocarcinoma (PDAC) cells usually overexpress the epidermal growth factor receptor (EGFR); however, most are resistant to the anti-EGFR monoclonal antibody, cetuximab. In this study, we report that the molecular mechanism of resistance to cetuximab in PDAC cells is mediated by the overexpression of active integrin β1 with downstream Src-Akt activation; this triggers an EGFR ligand-independent proliferation signaling, bypassing EGFR-blocking effect. Knockdown of integrin β1 or inhibition of Src or Akt sensitized cetuximab-resistant (CtxR) PDAC cells to cetuximab. We found that neuropilin-1 (NRP1) physically interacts with active integrin β1, but not inactive one, on the cell surface. To inhibit active integrin β1-driven signaling by targeting NRP1, while suppressing EGFR signaling, we generated an EGFR and NRP1 dual targeting antibody, Ctx-TPP11, by genetic fusion of the NRP1-targeting peptide, TPP11, to the C terminus of the cetuximab heavy chain (Ctx-TPP11). We demonstrate that Ctx-TPP11 efficiently inhibited the growth of CtxR PDAC cells, in vitro and in vivo. The sensitization mechanism involved downregulating active integrin β1 levels through NRP1-coupled internalization mediated by the TPP11 moiety, leading to the inhibition of active integrin β1-driven bypass signaling. Our findings identify aberrant active integrin β1-driven Src-Akt hyperactivation as a primary resistance mechanism to cetuximab in PDAC cells and offer an effective therapeutic strategy to overcome this resistance using an EGFR and NRP1 dual targeting antibody.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Garrido-Laguna I, Hidalgo M . Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol 2015; 12: 319–334.
Philip PA, Benedetti J, Corless CL, Wong R, O'Reilly EM, Flynn PJ et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol 2010; 28: 3605–3610.
Chong CR, Janne PA . The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med 2013; 19: 1389–1400.
Oliveira-Cunha M, Newman WG, Siriwardena AK . Epidermal growth factor receptor in pancreatic cancer. Cancers (Basel) 2011; 3: 1513–1526.
Wong MH, Xue A, Julovi SM, Pavlakis N, Samra JS, Hugh TJ et al. Cotargeting of epidermal growth factor receptor and PI3K overcomes PI3K-Akt oncogenic dependence in pancreatic ductal adenocarcinoma. Clin Cancer Res 2014; 20: 4047–4058.
Wheeler DL, Dunn EF, Harari PM . Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat Rev Clin Oncol 2010; 7: 493–507.
Larbouret C, Gaborit N, Chardes T, Coelho M, Campigna E, Bascoul-Mollevi C et al. In pancreatic carcinoma, dual EGFR/HER2 targeting with cetuximab/trastuzumab is more effective than treatment with trastuzumab/erlotinib or lapatinib alone: implication of receptors' down-regulation and dimers' disruption. Neoplasia 2012; 14: 121–130.
Li B, Meng Y, Zheng L, Zhang X, Tong Q, Tan W et al. Bispecific antibody to ErbB2 overcomes trastuzumab resistance through comprehensive blockade of ErbB2 heterodimerization. Cancer Res 2013; 73: 6471–6483.
Prud'homme GJ, Glinka Y . Neuropilins are multifunctional coreceptors involved in tumor initiation, growth, metastasis and immunity. Oncotarget 2012; 3: 921–939.
Guo HF, Vander Kooi CW . Neuropilin functions as an essential cell surface receptor. J Biol Chem 2015; 290: 29120–29126.
Pan Q, Chanthery Y, Liang WC, Stawicki S, Mak J, Rathore N et al. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell 2007; 11: 53–67.
Sugahara KN, Braun GB, de Mendoza TH, Kotamraju VR, French RP, Lowy AM et al. Tumor-penetrating iRGD peptide inhibits metastasis. Mol Cancer Ther 2015; 14: 120–128.
Jia H, Cheng L, Tickner M, Bagherzadeh A, Selwood D, Zachary I . Neuropilin-1 antagonism in human carcinoma cells inhibits migration and enhances chemosensitivity. Br J Cancer 2010; 102: 541–552.
Shin TH, Sung ES, Kim YJ, Kim KS, Kim SH, Kim SK et al. Enhancement of the tumor penetration of monoclonal antibody by fusion of a neuropilin-targeting peptide improves the antitumor efficacy. Mol Cancer Ther 2014; 13: 651–661.
Kim YJ, Bae J, Shin TH, Kang SH, Jeong M, Han Y et al. Immunoglobulin Fc-fused, neuropilin-1-specific peptide shows efficient tumor tissue penetration and inhibits tumor growth via anti-angiogenesis. J Control Release 2015; 216: 56–68.
Fukahi K, Fukasawa M, Neufeld G, Itakura J, Korc M . Aberrant expression of neuropilin-1 and -2 in human pancreatic cancer cells. Clin Cancer Res 2004; 10: 581–590.
Matsushita A, Gotze T, Korc M . Hepatocyte growth factor-mediated cell invasion in pancreatic cancer cells is dependent on neuropilin-1. Cancer Res 2007; 67: 10309–10316.
Wey JS, Gray MJ, Fan F, Belcheva A, McCarty MF, Stoeltzing O et al. Overexpression of neuropilin-1 promotes constitutive MAPK signalling and chemoresistance in pancreatic cancer cells. Br J Cancer 2005; 93: 233–241.
Zeng F, Luo F, Lv S, Zhang H, Cao C, Chen X et al. A monoclonal antibody targeting neuropilin-1 inhibits adhesion of MCF7 breast cancer cells to fibronectin by suppressing the FAK/p130cas signaling pathway. Anticancer Drugs 2014; 25: 663–672.
Valdembri D, Caswell PT, Anderson KI, Schwarz JP, Konig I, Astanina E et al. Neuropilin-1/GIPC1 signaling regulates alpha5beta1 integrin traffic and function in endothelial cells. PLoS Biol 2009; 7: e25.
Fukasawa M, Matsushita A, Korc M . Neuropilin-1 interacts with integrin beta1 and modulates pancreatic cancer cell growth, survival and invasion. Cancer Biol Ther 2007; 6: 1173–1180.
Kanda R, Kawahara A, Watari K, Murakami Y, Sonoda K, Maeda M et al. Erlotinib resistance in lung cancer cells mediated by integrin beta1/Src/Akt-driven bypass signaling. Cancer Res 2013; 73: 6243–6253.
Seguin L, Desgrosellier JS, Weis SM, Cheresh DA . Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol 2015; 25: 234–240.
Hou S, Isaji T, Hang Q, Im S, Fukuda T, Gu J . Distinct effects of beta1 integrin on cell proliferation and cellular signaling in MDA-MB-231 breast cancer cells. Sci Rep 2016; 6: 18430.
Arnoletti JP, Buchsbaum DJ, Huang ZQ, Hawkins AE, Khazaeli MB, Kraus MH et al. Mechanisms of resistance to Erbitux (anti-epidermal growth factor receptor) combination therapy in pancreatic adenocarcinoma cells. J Gastrointest Surg 2004; 8: 960–969 discussion 969–970.
Fotopoulou C, Baumunk D, Schmidt SC, Schumacher G . Additive growth inhibition after combined treatment of 2-methoxyestradiol and conventional chemotherapeutic agents in human pancreatic cancer cells. Anticancer Res 2010; 30: 4619–4624.
Iida M, Brand TM, Campbell DA, Li C, Wheeler DL . Yes and Lyn play a role in nuclear translocation of the epidermal growth factor receptor. Oncogene 2013; 32: 759–767.
Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ . c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem 1999; 274: 8335–8343.
Sung ES, Park KJ, Lee SH, Jang YS, Park SK, Park YH et al. A novel agonistic antibody to human death receptor 4 induces apoptotic cell death in various tumor cells without cytotoxicity in hepatocytes. Mol Cancer Ther 2009; 8: 2276–2285.
Arjonen A, Alanko J, Veltel S, Ivaska J . Distinct recycling of active and inactive beta1 integrins. Traffic 2012; 13: 610–625.
De Franceschi N, Hamidi H, Alanko J, Sahgal P, Ivaska J . Integrin traffic - the update. J Cell Sci 2015; 128: 839–852.
Luque A, Gomez M, Puzon W, Takada Y, Sanchez-Madrid F, Cabanas C . Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355-425) of the common beta 1 chain. J Biol Chem 1996; 271: 11067–11075.
Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM et al. Talin binding to integrin beta tails: a final common step in integrin activation. Science 2003; 302: 103–106.
Ye F, Kim C, Ginsberg MH . Reconstruction of integrin activation. Blood 2012; 119: 26–33.
Cox AD, Fesik SW, Kimmelman AC, Luo J, Der CJ . Drugging the undruggable RAS: mission possible? Nat Rev Drug Discov 2014; 13: 828–851.
Yaqoob U, Cao S, Shergill U, Jagavelu K, Geng Z, Yin M et al. Neuropilin-1 stimulates tumor growth by increasing fibronectin fibril assembly in the tumor microenvironment. Cancer Res 2012; 72: 4047–4059.
Okon IS, Coughlan KA, Zhang C, Moriasi C, Ding Y, Song P et al. Protein kinase LKB1 promotes RAB7-mediated neuropilin-1 degradation to inhibit angiogenesis. J Clin Invest 2014; 124: 4590–4602.
Choi DK, Bae J, Shin SM, Shin JY, Kim S, Kim YS . A general strategy for generating intact, full-length IgG antibodies that penetrate into the cytosol of living cells. MAbs 2014; 6: 1402–1414.
Choi HJ, Kim YJ, Lee S, Kim YS . A heterodimeric Fc-based bispecific antibody simultaneously targeting VEGFR-2 and Met exhibits potent antitumor activity. Mol Cancer Ther 2013; 12: 2748–2759.
Nguyen DH, Taub D . CXCR4 function requires membrane cholesterol: implications for HIV infection. J Immunol 2002; 168: 4121–4126.
Acknowledgements
This work was supported by grants from the Mid-career Researcher Program (2016R1A2A2A05005108) and the Pioneer Research Center Program (2014M3C1A3051470) from the National Research Foundation funded by the Korean government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Kim, YJ., Jung, K., Baek, DS. et al. Co-targeting of EGF receptor and neuropilin-1 overcomes cetuximab resistance in pancreatic ductal adenocarcinoma with integrin β1-driven Src-Akt bypass signaling. Oncogene 36, 2543–2552 (2017). https://doi.org/10.1038/onc.2016.407
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.407
This article is cited by
-
Integrinβ-1 in disorders and cancers: molecular mechanisms and therapeutic targets
Cell Communication and Signaling (2024)
-
Functional roles of SRC signaling in pancreatic cancer: Recent insights provide novel therapeutic opportunities
Oncogene (2023)
-
NRP1 inhibition modulates radiosensitivity of medulloblastoma by targeting cancer stem cells
Cancer Cell International (2022)
-
Ablation of neuropilin-1 improves the therapeutic response in conventional drug-resistant glioblastoma multiforme
Oncogene (2020)
-
The miR-141/neuropilin-1 axis is associated with the clinicopathology and contributes to the growth and metastasis of pancreatic cancer
Cancer Cell International (2019)