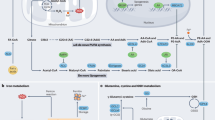
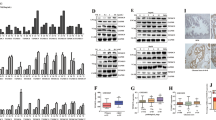
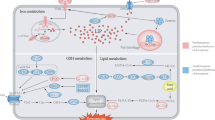
Abstract
A mounting body of evidence suggests that increased production of reactive oxygen species (ROS) is linked to aging processes and to the etiopathogenesis of aging-related diseases, such as cancer, diabetes, atherosclerosis and degenerative diseases like Parkinson’s and Alzheimer’s. Excess ROS are deleterious to normal cells, while in cancer cells, they can lead to accelerated tumorigenesis. In prostate cancer (PC), oxidative stress, an innate key event characterized by supraphysiological ROS concentrations, has been identified as one of the hallmarks of the aggressive disease phenotype. Specifically, oxidative stress is associated with PC development, progression and the response to therapy. Nevertheless, a thorough understanding of the relationships between oxidative stress, redox homeostasis and the activation of proliferation and survival pathways in healthy and malignant prostate remains elusive. Moreover, the failure of chemoprevention strategies targeting oxidative stress reduced the level of interest in the field after the recent negative results of the Selenium and Vitamin E Cancer Prevention Trial (SELECT) trial. Therefore, a revisit of the concept is warranted and several key issues need to be addressed: The consequences of changes in ROS levels with respect to altered redox homeostasis and redox-regulated processes in PC need to be established. Similarly, the key molecular events that cause changes in the generation of ROS in PC and the role for therapeutic strategies aimed at ameliorating oxidative stress need to be identified. Moreover, the issues whether genetic/epigenetic susceptibility for oxidative stress-induced prostatic carcinogenesis is an individual phenomenon and what measurements adequately quantify prostatic oxidative stress are also crucial. Addressing these matters will provide a more rational basis to improve the design of redox-related clinical trials in PC. This review summarizes accepted concepts and principles in redox research, and explores their implications and limitations in PC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Droge W . Free radicals in the physiological control of cell function. Physiol Rev 2002; 82: 47–95.
Winterbourn CC . Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol 2008; 4: 278–286.
Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK . Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res 2008; 68: 1777–1785.
Hirst DG, Robson T . Nitrosative stress in cancer therapy. Front Biosci 2007; 12: 3406–3418.
Murphy MP . How mitochondria produce reactive oxygen species. Biochem J 2009; 417: 1–13.
Schewe T . 15-Lipoxygenase-1: a prooxidant enzyme. Biol Chem 2002; 383: 365–374.
Bedard K, Krause KH . The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 2007; 87: 245–313.
Lu JP, Monardo L, Bryskin I, Hou ZF, Trachtenberg J, Wilson BC et al. Androgens induce oxidative stress and radiation resistance in prostate cancer cells though NADPH oxidase. Prostate Cancer Prostatic Dis 2010; 13: 39–46.
Khandrika L, Kumar B, Koul S, Maroni P, Koul HK . Oxidative stress in prostate cancer. Cancer Lett 2009; 282: 125–136.
Gupta-Elera G, Garrett AR, Robison RA, O'Neill KL . The role of oxidative stress in prostate cancer. Eur J Cancer Prev 2012; 21: 155–162.
Bostwick DG, Burke HB, Djakiew D, Euling S, Ho SM, Landolph J et al. Human prostate cancer risk factors. Cancer 2004; 101 (Suppl): 2371–2490.
Kroemer G . Mitochondria in cancer. Oncogene 2006; 25: 4630–4632.
Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J . Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem 2004; 266: 37–56.
Finkel T, Serrano M, Blasco MA . The common biology of cancer and ageing. Nature 2007; 448: 767–774.
Karayi MK, Markham AF . Molecular biology of prostate cancer. Prostate Cancer Prostatic Dis 2004; 7: 6–20.
Cazares LH, Drake RR, Esquela-Kirscher A, Lance RS, Semmes OJ, Troyer DA . Molecular pathology of prostate cancer. Cancer Biomark 2010; 9: 441–459.
Lin X, Asgari K, Putzi MJ, Gage WR, Yu X, Cornblatt BS et al. Reversal of GSTP1 CpG island hypermethylation and reactivation of pi-class glutathione S-transferase (GSTP1) expression in human prostate cancer cells by treatment with procainamide. Cancer Res 2001; 61: 8611–8616.
Yu S, Khor TO, Cheung KL, Li W, Wu TY, Huang Y et al. Nrf2 expression is regulated by epigenetic mechanisms in prostate cancer of TRAMP mice. PLoS One 2010; 5: e8579.
Frohlich DA, McCabe MT, Arnold RS, Day ML . The role of Nrf2 in increased reactive oxygen species and DNA damage in prostate tumorigenesis. Oncogene 2008; 27: 4353–4362.
Kobayashi M, Yamamoto M . Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal 2005; 7: 385–394.
Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 1997; 236: 313–322.
Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S . Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res 2002; 62: 5196–5203.
Costello LC, Franklin RB . The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Mol Cancer 2006; 5: 17.
Franklin RB, Milon B, Feng P, Costello LC . Zinc and zinc transporters in normal prostate and the pathogenesis of prostate cancer. Front Biosci 2005; 10: 2230–2239.
Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS et al. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J Neurosci 2004; 24: 7779–7788.
Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS . Calcium, ATP, and ROS: a mitochondrial love–hate triangle. Am J Physiol Cell Physiol 2004; 287: C817–C833.
Harman D . Aging: a theory based on free radical and radiation chemistry. J Gerontol 1956; 11: 298–300.
Costello LC, Liu Y, Zou J, Franklin RB . Evidence for a zinc uptake transporter in human prostate cancer cells which is regulated by prolactin and testosterone. J Biol Chem 1999; 274: 17499–17504.
Whelan KF, Lu JP, Fridman E, Wolf A, Honig A, Paulin G et al. What can surrogate tissues tell us about the oxidative stress status of the prostate? A hypothesis-generating in vivo study. PLoS One 2010; 5: e15880.
Ripple MO, Henry WF, Rago RP, Wilding G . Prooxidant-antioxidant shift induced by androgen treatment of human prostate carcinoma cells. J Natl Cancer Inst 1997; 89: 40–48.
Sun XY, Donald SP, Phang JM . Testosterone and prostate specific antigen stimulate generation of reactive oxygen species in prostate cancer cells. Carcinogenesis 2001; 22: 1775–1780.
Pinthus JH, Bryskin I, Trachtenberg J, Lu JP, Singh G, Fridman E et al. Androgen induces adaptation to oxidative stress in prostate cancer: implications for treatment with radiation therapy. Neoplasia 2007; 9: 68–80.
Lin H, Lu JP, Laflamme P, Qiao S, Shayegan B, Bryskin I et al. Inter-related in vitro effects of androgens, fatty acids and oxidative stress in prostate cancer: a mechanistic model supporting prevention strategies. Int J Oncol 2010; 37: 761–766.
Pinthus JH, Lu JP, Bidaisee LA, Lin H, Bryskine I, Gupta RS et al. Androgen-dependent regulation of medium and long chain fatty acids uptake in prostate cancer. Prostate 2007; 67: 1330–1338.
Kikuchi H, Hikage M, Miyashita H, Fukumoto M . NADPH oxidase subunit, gp91(phox) homologue, preferentially expressed in human colon epithelial cells. Gene 2000; 254: 237–243.
Brar SS, Corbin Z, Kennedy TP, Hemendinger R, Thornton L, Bommarius B et al. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol Cell Physiol 2003; 285: C353–C369.
Tam NN, Gao Y, Leung YK, Ho SM . Androgenic regulation of oxidative stress in the rat prostate: involvement of NAD(P)H oxidases and antioxidant defense machinery during prostatic involution and regrowth. Am J Pathol 2003; 163: 2513–2522.
Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009; 373: 1083–1096.
Lu JP, Hou ZF, Duivenvoorden WC, Whelan K, Honig A, Pinthus JH . Adiponectin inhibits oxidative stress in human prostate carcinoma cells. Prostate Cancer Prostatic Dis 2012; 15: 28–35.
Masko EM, Allott EH, Freedland SJ . The relationship between nutrition and prostate cancer: is more always better? Eur Urol 2013; 63: 810–820.
Koutros S, Beane Freeman LE, Lubin JH, Heltshe SL, Andreotti G, Barry KH et al. Risk of total and aggressive prostate cancer and pesticide use in the agricultural health study. Am J Epidemiol 2013; 177: 59–74.
Santti R, Newbold RR, Makela S, Pylkkanen L, McLachlan JA . Developmental estrogenization and prostatic neoplasia. Prostate 1994; 24: 67–78.
Prins GS . Endocrine disruptors and prostate cancer risk. Endocr Relat Cancer 2008; 15: 649–656.
Felty Q, Xiong WC, Sun D, Sarkar S, Singh KP, Parkash J et al. Estrogen-induced mitochondrial reactive oxygen species as signal-transducing messengers. Biochemistry 2005; 44: 6900–6909.
Venditti P, Di Meo S . Thyroid hormone-induced oxidative stress. Cell Mol Life Sci 2006; 63: 414–434.
Stephens FO . Phytoestrogens and prostate cancer: possible preventive role. Med J Aust 1997; 167: 138–140.
Mishra SI, Dickerson V, Najm W . Phytoestrogens and breast cancer prevention: what is the evidence? Am J Obstet Gynecol 2003; 188 (Suppl): S66–S70.
Strom SS, Yamamura Y, Duphorne CM, Spitz MR, Babaian RJ, Pillow PC et al. Phytoestrogen intake and prostate cancer: a case-control study using a new database. Nutr Cancer 1999; 33: 20–25.
Coussens LM, Werb Z . Inflammation and cancer. Nature 2002; 420: 860–867.
Dennis LK, Lynch CF, Torner JC . Epidemiologic association between prostatitis and prostate cancer. Urology 2002; 60: 78–83.
Harris MT, Feldberg RS, Lau KM, Lazarus NH, Cochrane DE . Expression of proinflammatory genes during estrogen-induced inflammation of the rat prostate. Prostate 2000; 44: 19–25.
Klein EA, Casey G, Silverman R . Genetic susceptibility and oxidative stress in prostate cancer: integrated model with implications for prevention. Urology 2006; 68: 1145–1151.
Bostwick DG, Alexander EE, Singh R, Shan A, Qian J, Santella RM et al. Antioxidant enzyme expression and reactive oxygen species damage in prostatic intraepithelial neoplasia and cancer. Cancer 2000; 89: 123–134.
Oberley TD, Zhong W, Szweda LI, Oberley LW . Localization of antioxidant enzymes and oxidative damage products in normal and malignant prostate epithelium. Prostate 2000; 44: 144–155.
Donkena KV, Young CY, Tindall DJ . Oxidative stress and DNA methylation in prostate cancer. Obstet Gynecol Int 2010; 2010: 302051.
Tyurina YY, Tyurin VA, Epperly MW, Greenberger JS, Kagan VE . Oxidative lipidomics of gamma-irradiation-induced intestinal injury. Free Radic Biol Med 2008; 44: 299–314.
Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P . Redox regulation of cell survival. Antioxid Redox Signal 2008; 10: 1343–1374.
Poole LB, Karplus PA, Claiborne A . Protein sulfenic acids in redox signaling. Annu Rev Pharmacol Toxicol 2004; 44: 325–347.
Ali FE, Barnham KJ, Barrow CJ, Separovic F . Metal catalyzed oxidation of tyrosine residues by different oxidation systems of copper/hydrogen peroxide. J Inorg Biochem 2004; 98: 173–184.
Suzuki YJ, Carini M, Butterfield DA . Protein carbonylation. Antioxid Redox Signal 2010; 12: 323–325.
Turpaev KT . Reactive oxygen species and regulation of gene expression. Biochemistry (Moscow) 2002; 67: 281–292.
England K, Cotter TG . Direct oxidative modifications of signalling proteins in mammalian cells and their effects on apoptosis. Redox Rep 2005; 10: 237–245.
Cross JV, Templeton DJ . Regulation of signal transduction through protein cysteine oxidation. Antioxid Redox Signal 2006; 8: 1819–1827.
Cho SH, Lee CH, Ahn Y, Kim H, Kim H, Ahn CY et al. Redox regulation of PTEN and protein tyrosine phosphatases in H(2)O(2) mediated cell signaling. FEBS Lett 2004; 560: 7–13.
Squier TC . Redox modulation of cellular metabolism through targeted degradation of signaling proteins by the proteasome. Antioxid Redox Signal 2006; 8: 217–228.
de Bruin EC, Medema JP . Apoptosis and non-apoptotic deaths in cancer development and treatment response. Cancer Treat Rev 2008; 34: 737–749.
Simon HU, Haj-Yehia A, Levi-Schaffer F . Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000; 5: 415–418.
Shiota M, Yokomizo A, Naito S . Pro-survival and anti-apoptotic properties of androgen receptor signaling by oxidative stress promote treatment resistance in prostate cancer. Endocr Relat Cancer 2012; 19: R243–R253.
Kongara S, Karantza V . The interplay between autophagy and ROS in tumorigenesis. Front Oncol 2012; 2: 171.
Mohamed MM, Sloane BF . Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer 2006; 6: 764–775.
Fernandez PL, Farre X, Nadal A, Fernandez E, Peiro N, Sloane BF et al. Expression of cathepsins B and S in the progression of prostate carcinoma. Int J Cancer 2001; 95: 51–55.
Plaks V, Posen Y, Mazor O, Brandis A, Scherz A, Salomon Y . Homologous adaptation to oxidative stress induced by the photosensitized Pd-bacteriochlorophyll derivative (WST11) in cultured endothelial cells. J Biol Chem 2004; 279: 45713–45720.
Pagliarulo V, Bracarda S, Eisenberger MA, Mottet N, Schroder FH, Sternberg CN et al. Contemporary role of androgen deprivation therapy for prostate cancer. Eur Urol 2012; 61: 11–25.
Tabassum A, Bristow RG, Venkateswaran V . Ingestion of selenium and other antioxidants during prostate cancer radiotherapy: a good thing? Cancer Treat Rev 2010; 36: 230–234.
Steinberg J, Oyasu R, Lang S, Sintich S, Rademaker A, Lee C et al. Intracellular levels of SGP-2 (Clusterin) correlate with tumor grade in prostate cancer. Clin Cancer Res 1997; 3: 1707–1711.
July LV, Akbari M, Zellweger T, Jones EC, Goldenberg SL, Gleave ME . Clusterin expression is significantly enhanced in prostate cancer cells following androgen withdrawal therapy. Prostate 2002; 50: 179–188.
Djeu JY, Wei S . Clusterin and chemoresistance. Adv Cancer Res 2009; 105: 77–92.
Zellweger T, Kiyama S, Chi K, Miyake H, Adomat H, Skov K et al. Overexpression of the cytoprotective protein clusterin decreases radiosensitivity in the human LNCaP prostate tumour model. BJU Int 2003; 92: 463–469.
Miyake H, Hara I, Gleave ME, Eto H . Protection of androgen-dependent human prostate cancer cells from oxidative stress-induced DNA damage by overexpression of clusterin and its modulation by androgen. Prostate 2004; 61: 318–323.
Shiota M, Zoubeidi A, Kumano M, Beraldi E, Naito S, Nelson CC et al. Clusterin is a critical downstream mediator of stress-induced YB-1 transactivation in prostate cancer. Mol Cancer Res 2011; 9: 1755–1766.
Klein EA, Thompson IM Jr., Tangen CM, Crowley JJ, Lucia MS, Goodman PJ et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011; 306: 1549–1556.
Moyad MA . Selenium and vitamin E supplements for prostate cancer: evidence or embellishment? Urology 2002; 59 (Suppl 1): 9–19.
Yang CS, Suh N, Kong AN . Does vitamin E prevent or promote cancer? Cancer Prev Res (Philadelphia) 2012; 5: 701–705.
Martinez EE, Anderson PD, Logan M, Abdulkadir SA . Antioxidant treatment promotes prostate epithelial proliferation in Nkx3.1 mutant mice. PLoS One 2012; 7: e46792.
Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN . Bilirubin is an antioxidant of possible physiological importance. Science 1987; 235: 1043–1046.
Ames BN, Cathcart R, Schwiers E, Hochstein P . Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA 1981; 78: 6858–6862.
Winterbourn CC, Hampton MB . Thiol chemistry and specificity in redox signaling. Free Radic Biol Med 2008; 45: 549–561.
McBean GJ, Flynn J . Molecular mechanisms of cystine transport. Biochem Soc Trans 2001; 29 (Part 6): 717–722.
Barber NJ, Barber J . Lycopene and prostate cancer. Prostate Cancer Prostatic Dis 2002; 5: 6–12.
Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC . Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst 1995; 87: 1767–1776.
Constantinou C, Hyatt JA, Vraka PS, Papas A, Papas KA, Neophytou C et al. Induction of caspase-independent programmed cell death by vitamin E natural homologs and synthetic derivatives. Nutr Cancer 2009; 61: 864–874.
Moghadaszadeh B, Beggs AH . Selenoproteins and their impact on human health through diverse physiological pathways. Physiology (Bethesda, MD) 2006; 21: 307–315.
Galati G, O'Brien PJ . Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic Biol Med 2004; 37: 287–303.
Veech RL . The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids 2004; 70: 309–319.
Mavropoulos JC, Isaacs WB, Pizzo SV, Freedland SJ . Is there a role for a low-carbohydrate ketogenic diet in the management of prostate cancer? Urology 2006; 68: 15–18.
Hoque A, Ambrosone CB, Till C, Goodman PJ, Tangen C, Kristal A et al. Serum oxidized protein and prostate cancer risk within the Prostate Cancer Prevention Trial. Cancer Prev Res (Philadelphia) 2010; 3: 478–483.
Marshall JR, Tangen CM, Sakr WA, Wood DP Jr., Berry DL, Klein EA et al. Phase III trial of selenium to prevent prostate cancer in men with high-grade prostatic intraepithelial neoplasia: SWOG S9917. Cancer Prev Res (Philadelphia) 2011; 4: 1761–1769.
Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen J et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA 2009; 301: 52–62.
Heinonen OP, Albanes D, Virtamo J, Taylor PR, Huttunen JK, Hartman AM et al. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst 1998; 90: 440–446.
Meyer F, Galan P, Douville P, Bairati I, Kegle P, Bertrais S et al. Antioxidant vitamin and mineral supplementation and prostate cancer prevention in the SU.VI.MAX trial. Int J Cancer 2005; 116: 182–186.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paschos, A., Pandya, R., Duivenvoorden, W. et al. Oxidative stress in prostate cancer: changing research concepts towards a novel paradigm for prevention and therapeutics. Prostate Cancer Prostatic Dis 16, 217–225 (2013). https://doi.org/10.1038/pcan.2013.13
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2013.13
Keywords
This article is cited by
-
Atopic allergic conditions and prostate cancer risk and survival in the Multiethnic Cohort study
British Journal of Cancer (2023)
-
Effect of γ-Al2O3 Nanostructure Surface Morphology on Lipid Peroxidation and Cathepsin Activity in Neuro-2a Neuroblastoma Cells
Journal of Cluster Science (2023)
-
Acidic Exo-Polysaccharide Obtained from Bacillus sp. NRC5 Attenuates Testosterone-DMBA-Induced Prostate Cancer in Rats via Inhibition of 5 α-Reductase and Na+/K+ ATPase Activity Mechanisms
Current Microbiology (2023)
-
Comparing histology between prostate cognitive fusion targeted biopsy and radical prostatectomy: exploring risk factors of Gleason score upgrading in Chinese patients
Journal of Cancer Research and Clinical Oncology (2023)
-
PGC1 alpha coactivates ERG fusion to drive antioxidant target genes under metabolic stress
Communications Biology (2022)