Abstract
Background/Objectives
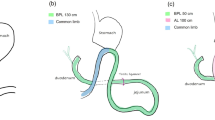
Changes in gut hormone secretion are important for the anti-diabetic effects of bariatric surgery. Roux-en-Y gastric bypass (RYGB) with extended biliopancreatic limb (BPL) length may improve the metabolic outcomes when compared to the classical procedure. The purpose of this study was to compare the gut hormone responses to a liquid mixed meal after RYGB with one of two different BPL lengths.
Subjects/Methods
Non-diabetic weight-stable individuals previously submitted to classical RYGB (n = 9; BPL length: 87.8 ± 20.5 cm) or long BPL RYGB (n = 11; BPL length: 200 cm) underwent a liquid mixed-meal tolerance test (MMTT). Blood was sampled at baseline and 15, 30, 45, 60, 90 and 120 min later for measurement of plasma glucose, enteropancreatic hormones and total bile acids (TBA).
Results
Plasma glucose excursion curves were similar in the two groups. The long BPL RYGB group displayed significantly higher fasting and post-prandial GLP-1 (t = 0 min, p = 0.01 and t = 45 min, p < 0.05; tAUC: 11,205 ± 3399 vs 7889 ± 1686 pmol/L × min, p = 0.02) and neurotensin (t = 0 min, p = 0.02; t = 45 min, p < 0.05 and t = 60 min, p < 0.01; tAUC: 18,392 ± 7066 vs 11,437 ± 3658 pmol/L × min, p = 0.02) levels, while responses of GIP (t = 15 min, p < 0.01), insulin and C-peptide (t = 30 min, p < 0.001) were lower as compared to classical RYGB. There were no differences in glucagon, PP, PYY and TBA between the groups.
Conclusions
RYGB with a longer BPL results in a distinctive post-prandial hormone profile with augmented GLP-1 and neurotensin responses that could be beneficial for the metabolic outcomes of the surgery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Yan Y, Sha Y, Yao G, Wang S, Kong F, Liu H, et al. Roux-en-Y gastric bypass versus medical treatment for type 2 diabetes mellitus in obese patients: a systematic review and meta-analysis of randomized controlled trials. Medicine. 2016;95:e3462.
Kissler HJ, Settmacher U. Bariatric surgery to treat obesity. Semin Nephrol. 2013;33:75–89.
Nora M, Guimaraes M, Almeida R, Martins P, Goncalves G, Freire MJ, et al. Metabolic laparoscopic gastric bypass for obese patients with type 2 diabetes. Obes Surg. 2011;21:1643–9.
Madsbad S, Dirksen C, Holst JJ. Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. Lancet Diabetes Endocrinol. 2014;2:152–64.
Berbiglia L, Zografakis JG, Dan AG. Laparoscopic Roux-en-Y gastric bypass: surgical technique and perioperative care. Surg Clin North Am. 2016;96:773–94.
Williams KV, Kelley DE. Metabolic consequences of weight loss on glucose metabolism and insulin action in type 2 diabetes. Diabetes Obes Metab. 2000;2:121–9.
Nelson DW, Blair KS, Martin MJ. Analysis of obesity-related outcomes and bariatric failure rates with the duodenal switch vs gastric bypass for morbid obesity. Arch Surg. 2012;147:847–54.
Sala PC, Torrinhas RS, Heymsfield SB, Waitzberg DL. Type 2 diabetes mellitus: a possible surgically reversible intestinal dysfunction. Obes Surg. 2012;22:167–76.
Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–9.
Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89:2608–15.
Sala PC, Torrinhas RS, Giannella-Neto D, Waitzberg DL. Relationship between gut hormones and glucose homeostasis after bariatric surgery. Diabetol Metab Syndr. 2014;6:87.
Holst JJ. Gut hormones and gastric bypass. Cardiovasc Endocrinol. 2016;5:69–74.
Jacobsen SH, Olesen SC, Dirksen C, Jorgensen NB, Bojsen-Moller KN, Kielgast U, et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg. 2012;22:1084–96.
Dirksen C, Jorgensen NB, Bojsen-Moller KN, Kielgast U, Jacobsen SH, Clausen TR, et al. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. Int J Obes (Lond). 2013;37:1452–9.
le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780–5.
Nergaard BJ, Leifsson BG, Hedenbro J, Gislason H. Gastric bypass with long alimentary limb or long pancreato-biliary limb—long-term results on weight loss, resolution of co-morbidities and metabolic parameters. Obes Surg. 2014;24:1595–602.
Nora M, Morais T, Almeida R, Guimaraes M, Monteiro MP. Should Roux-en-Y gastric bypass biliopancreatic limb length be tailored to achieve improved diabetes outcomes? Medicine. 2017;96:e8859.
Fried M, Yumuk V, Oppert JM, Scopinaro N, Torres A, Weiner R, et al. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Surg. 2014;24:42–55.
Orskov C, Rabenhoj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes. 1994;43:535–9.
Krarup T, Holst JJ. The heterogeneity of gastric inhibitory polypeptide in porcine and human gastrointestinal mucosa evaluated with five different antisera. Regul Pept. 1984;9:35–46.
Orskov C, Jeppesen J, Madsbad S, Holst JJ. Proglucagon products in plasma of noninsulin-dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. J Clin Invest. 1991;87:415–23.
Toräng S, Bojsen-Moller KN, Svane MS, Hartmann B, Rosenkilde MM, Madsbad S et al. In vivo and in vitro degradation of peptide YY3-363-36 to inactive peptide YY3-343-34 in humans. Am J Physiol Regul Integr Comp Physiol. 2016;310:R866-R874.
Kuhre RE, Bechmann LE, Wewer Albrechtsen NJ, Hartmann B, Holst JJ. Glucose stimulates neurotensin secretion from the rat small intestine by mechanisms involving SGLT1 and GLUT2, leading to cell depolarization and calcium influx. Am J Physiol Endocrinol Metab. 2015;308:E1123–30.
Pedersen JH, Stadil F, Fahrenkrug J. Preparation of 125I-(Tyr 3)- and 125I-(Tyr 11)- neurotensin for radioimmunoassay. Scand J Clin Lab Invest. 1983;43:483–91.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Bojsen-Moller KN, Dirksen C,Svane MS, Jorgensen NB, Holst JJ, Richter EA, et al. Variable reliability of surrogate measures of insulin sensitivity after Roux-en-Y gastric bypass. Am J Physiol Regul Integr Comp Physiol. 2017;312:R797–R805.
Meek CL, Lewis HB, Reimann F, Gribble FM, Park AJ. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides. 2016;77:28–37.
Stefanidis D, Kuwada TS, Gersin KS. The importance of the length of the limbs for gastric bypass patients--an evidence-based review. Obes Surg. 2011;21:119–24.
Kaska Ł, Kobiela J, Proczko M, Stefaniak T, Śledziński Z. Does the length of the biliary limb influence medium-term laboratory remission of type 2 diabetes mellitus after Roux-en-Y gastric bypass in morbidly obese patients? Wideochir Inne Tech Maloinwazyjne. 2014;9:31–9.
Freckmann G, Hagenlocher S, Baumstark A, Jendrike N, Gillen RC, Rössner K, et al. Continuous glucose profiles in healthy subjects under everyday life conditions and after different meals. J Diabetes Sci Technol. 2007;1:695–703.
Triplitt CL. Examining the mechanisms of glucose regulation. Am J Manag Care. 2012;18(1 Suppl):S4–10.
Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–42.
Salinari S, Carr RD, Guidone C, Bertuzzi A, Cercone S, Riccioni ME, et al. Nutrient infusion bypassing duodenum-jejunum improves insulin sensitivity in glucose-tolerant and diabetic obese subjects. Am J Physiol Endocrinol Metab. 2013;305:E59–66.
Guedes TP, Martins S, Costa M, Pereira SS, Morais T, Santos A, et al. Detailed characterization of incretin cell distribution along the human small intestine. Surg Obes Relat Dis. 2015;11:1323–31.
Jorgensen NB, Jacobsen SH, Dirksen C, Bojsen-Moller KN, Naver L, Hvolris L, et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab. 2012;303:E122–31.
McIntosh CH, Widenmaier S, Kim SJ. Glucose-dependent insulinotropic polypeptide (gastric inhibitory polypeptide; GIP). Vitam Horm. 2009;80:409–71.
Goldfine AB, Mun EC, Devine E, Bernier R, Baz-Hecht M, Jones DB, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92:4678–85.
Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601.
Dirksen C, Jorgensen NB, Bojsen-Moller KN, Jacobsen SH, Hansen DL, Worm D, et al. Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Diabetologia. 2012;55:1890–901.
Vilsboll T, Krarup T, Madsbad S, Holst JJ. Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regul Pept. 2003;114:115–21.
Monteiro MP, Batterham RL. The importance of the gastrointestinal tract in controlling food intake and regulating energy balance. Gastroenterology. 2017;152:1707–17 e2.
Grunddal KV, Ratner CF, Svendsen B, Sommer F, Engelstoft MS, Madsen AN, et al. Neurotensin is coexpressed, coreleased, and acts together with GLP-1 and PYY in enteroendocrine control of metabolism. Endocrinology. 2016;157:176–94.
Weiss HG, Nehoda H, Labeck B, Hourmont K, Marth C, Aigner F. Pregnancies after adjustable gastric banding. Obes Surg. 2001;11:303–6.
Holdstock C, Zethelius B, Sundbom M, Karlsson FA, Eden Engstrom B. Postprandial changes in gut regulatory peptides in gastric bypass patients. Int J Obes (Lond). 2008;32:1640–6.
Ratner C, Skov LJ, Raida Z, Bachler T, Bellmann-Sickert K, Le Foll C, et al. Effects of peripheral neurotensin on appetite regulation and its role in gastric bypass surgery. Endocrinology. 2016;157:3482–92.
Lund A, Bagger JI, Wewer Albrechtsen NJ, Christensen M, Grøndahl M, Hartmann B et al. Evidence of extrapancreatic glucagon secretion in man. Diabetes. 2016;65:585-597.
Holst JJ, Pedersen JH, Baldissera F, Stadil F. Circulating glucagon after total pancreatectomy in man. Diabetologia. 1983;25:396–9.
Geary N. Effects of glucagon, insulin, amylin and CGRP on feeding. Neuropeptides. 1999;33:400–5.
Salem V, Izzi-Engbeaya C, Coello C, Thomas DB, Chambers ES, Comninos AN, et al. Glucagon increases energy expenditure independently of brown adipose tissue activation in humans. Diabetes Obes Metab. 2016;18:72–81.
Nannipieri M, Baldi S, Mari A, Colligiani D, Guarino D, Camastra S, et al. Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. J Clin Endocrinol Metab. 2013;98:4391–9.
Schrumpf E, Linnestad P, Nygaard K, Giercksky KE, Fausa O. Pancreatic polypeptide secretion before and after gastric bypass surgery for morbid obesity. Scand J Gastroenterol. 1981;16:1009–14.
Miyachi T, Nagao M, Shibata C, Kitahara Y, Tanaka N, Watanabe K, et al. Biliopancreatic limb plays an important role in metabolic improvement after duodenal-jejunal bypass in a rat model of diabetes. Surgery. 2016;159:1360–71.
Risstad H, Kristinsson JA, Fagerland MW, le Roux CW, Birkeland KI, Gulseth HL, et al. Bile acid profiles over 5 years after gastric bypass and duodenal switch: results from a randomized clinical trial. Surg Obes Relat Dis. 2017;13:1544–53.
Brighton CA, Rievaj J, Kuhre RE, Glass LL, Schoonjans K, Holst JJ, et al. Bile acids trigger GLP-1 release predominantly by accessing basolaterally located G protein–coupled bile acid receptors. Endocrinology. 2015;156:3961–70.
Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring). 2009;17:1671–7.
Acknowledgements
Authors would like to thank Lene Brus Albaek from the Department of Biomedical Sciences, University of Copenhagen, Copenhagen, Denmark, and Lene Ravn from the Department of Clinical Biochemistry, Rigshospitalet, Copenhagen, Denmark, for outstanding technical assistance with assays.
Funding
UMIB is funded by grants from Foundation for Science and Technology (FCT) Portugal (PEst-OE/SAU/UI0215/2014) co-funded by FEDER funds through the Operational Programme Competitiveness Factors—COMPETE/QREN. This research is part of a larger project granted with the National Diabetes Award 2017 of the Portuguese Diabetes Society (SPD). JJH holds an unrestricted grant from the NNF Center for Basic Metabolic Research, Copenhagen, Denmark. The NNF foundation Center for Basic Metabolic Research is an independent research institution at the University of Copenhagen, Denmark.
Author Contributions
BGP, MG, JJH, MN and MPM planned and designed the study; BGP, MG, SV, BH, LH and MN conducted data acquisition; BGP, TM, MG, SV, BH, JJH, MN and MPM participated in analysis and interpretation of data; BGP and MPM wrote the manuscript; BGP, TM, MG, SV, BH, LH, JJH, MN and MPM revised the manuscript. All authors have approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Patrício, B.G., Morais, T., Guimarães, M. et al. Gut hormone release after gastric bypass depends on the length of the biliopancreatic limb. Int J Obes 43, 1009–1018 (2019). https://doi.org/10.1038/s41366-018-0117-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0117-y
This article is cited by
-
Individuals with type 2 diabetes have higher density of small intestinal neurotensin-expressing cells
Molecular and Cellular Biochemistry (2023)
-
Towards precision medicine in bariatric surgery prescription
Reviews in Endocrine and Metabolic Disorders (2023)
-
Similar Gut Hormone Secretions Two Years After One Anastomosis Gastric Bypass and Roux-en-Y Gastric Bypass: a Pilot Study
Obesity Surgery (2022)
-
Effects of short or long biliopancreatic limb length after laparoscopic Roux-en-Y gastric bypass surgery for obesity: a propensity score-matched analysis
Langenbeck's Archives of Surgery (2022)
-
Can Metabolite and Hormone Profiles Provide a Rationale for Choosing Between Bariatric Procedures?
Obesity Surgery (2021)