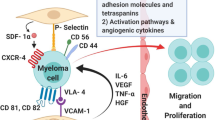
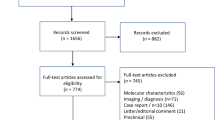
Abstract
Extramedullary multiple myeloma (EMM) is an aggressive subentity of multiple myeloma, characterized by the ability of a subclone to thrive and grow independent of the bone marrow microenvironment, resulting in a high-risk state associated with increased proliferation, evasion of apoptosis and treatment resistance. Despite improvement in survival for most patients with multiple myeloma over recent decades, outcomes are generally poor when EMM develops. Understanding the molecular underpinnings leading to homing of plasma cells in ecosystems outside the bone marrow will be crucial for therapeutically manipulating the microenvironment and targeting key signaling pathways. Herein, we discuss the evolutionary biology of EMM, underscore the importance of a uniform definition, discuss prognostic significance, and provide current and emerging treatment strategies for managing this rare subentity of multiple myeloma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–48.
Blade J, Fernandez de Larrea C, Rosinol L, Cibeira MT, Jimenez R, Powles R. Soft-tissue plasmacytomas in multiple myeloma: incidence, mechanisms of extramedullary spread, and treatment approach. J Clin Oncol. 2011;29:3805–12.
Vande Broek I, Vanderkerken K, Van Camp B, Van Riet I. Extravasation and homing mechanisms in multiple myeloma. Clin Exp Metastasis. 2008;25:325–34.
Weinstock M, Ghobrial IM. Extramedullary multiple myeloma. Leuk Lymphoma. 2013;54:1135–41.
Fernandez de Larrea C, Kyle RA, Durie BG, Ludwig H, Usmani S, Vesole DH, et al. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia. 2013;27:780–91.
International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–57.
Touzeau C, Moreau P. How I treat extramedullary myeloma. Blood. 2016;127:971–6.
Rasche L, Bernard C, Topp MS, Kapp M, Duell J, Wesemeier C, et al. Features of extramedullary myeloma relapse: high proliferation, minimal marrow involvement, adverse cytogenetics: a retrospective single-center study of 24 cases. Ann Hematol. 2012;91:1031–7.
Chang H, Qi X, Yeung J, Reece D, Xu W, Patterson B. Genetic aberrations including chromosome 1 abnormalities and clinical features of plasma cell leukemia. Leuk Res. 2009;33:259–62.
Cifola I, Lionetti M, Pinatel E, Todoerti K, Mangano E, Pietrelli A, et al. Whole-exome sequencing of primary plasma cell leukemia discloses heterogeneous mutational patterns. Oncotarget. 2015;6:17543–58.
Usmani SZ, Heuck C, Mitchell A, Szymonifka J, Nair B, Hoering A, et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over-represented in high-risk disease even in the era of novel agents. Haematologica. 2012;97:1761–7.
Pasmantier MW, Azar HA. Extraskeletal spread in multiple plasma cell myeloma. A review of 57 autopsied cases. Cancer. 1969;23:167–74.
Blade J, Lust JA, Kyle RA. Immunoglobulin D multiple myeloma: presenting features, response to therapy, and survival in a series of 53 cases. J Clin Oncol. 1994;12:2398–404.
Blade J, Kyle RA, Greipp PR. Presenting features and prognosis in 72 patients with multiple myeloma who were younger than 40 years. Br J Haematol. 1996;93:345–51.
Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010;21:325–30.
Pour L, Sevcikova S, Greslikova H, Kupska R, Majkova P, Zahradova L, et al. Soft-tissue extramedullary multiple myeloma prognosis is significantly worse in comparison to bone-related extramedullary relapse. Haematologica. 2014;99:360–4.
Batsukh K, Lee SE, Min GJ, Park SS, Jeon YW, Yoon JH, et al. Distinct clinical outcomes between paramedullary and extramedullary lesions in newly diagnosed multiple myeloma. Immune Netw. 2017;17:250–60.
Dawson MA, Patil S, Spencer A. Extramedullary relapse of multiple myeloma associated with a shift in secretion from intact immunoglobulin to light chains. Haematologica. 2007;92:143–4.
Creach KM, Foote RL, Neben-Wittich MA, Kyle RA. Radiotherapy for extramedullary plasmacytoma of the head and neck. Int J Radiat Oncol Biol Phys. 2009;73:789–94.
Dimopoulos MA, Hamilos G. Solitary bone plasmacytoma and extramedullary plasmacytoma. Curr Treat Options Oncol. 2002;3:255–9.
Ravi P, Kumar SK, Roeker L, Gonsalves W, Buadi F, Lacy MQ, et al. Revised diagnostic criteria for plasma cell leukemia: results of a Mayo Clinic study with comparison of outcomes to multiple myeloma. Blood Cancer J. 2018;8:116.
Tiedemann RE, Gonzalez-Paz N, Kyle RA, Santana-Davila R, Price-Troska T, Van Wier SA, et al. Genetic aberrations and survival in plasma cell leukemia. Leukemia. 2008;22:1044–52.
Garcia-Sanz R, Orfao A, Gonzalez M, Tabernero MD, Blade J, Moro MJ, et al. Primary plasma cell leukemia: clinical, immunophenotypic, DNA ploidy, and cytogenetic characteristics. Blood. 1999;93:1032–7.
Morgan GJ, Walker BA, Davies FE. The genetic architecture of multiple myeloma. Nat Rev Cancer. 2012;12:335–48.
Rasche L, Chavan SS, Stephens OW, Patel PH, Tytarenko R, Ashby C, et al. Spatial genomic heterogeneity in multiple myeloma revealed by multi-region sequencing. Nat Commun. 2017;8:268.
Pawlyn C, Morgan GJ. Evolutionary biology of high-risk multiple myeloma. Nat Rev Cancer. 2017;17:543–56.
Bergsagel PL, Kuehl WM, Zhan F, Sawyer J, Barlogie B, Shaughnessy J Jr. Cyclin D dysregulation: an early and unifying pathogenic event in multiple myeloma. Blood. 2005;106:296–303.
Keats JJ, Maxwell CA, Taylor BJ, Hendzel MJ, Chesi M, Bergsagel PL, et al. Overexpression of transcripts originating from the MMSET locus characterizes all t(4;14)(p16;q32)-positive multiple myeloma patients. Blood. 2005;105:4060–9.
Jelinek T, Bezdekova R, Zatopkova M, Burgos L, Simicek M, Sevcikova T, et al. Current applications of multiparameter flow cytometry in plasma cell disorders. Blood Cancer J. 2017;7:e617.
Jelinek T, Kryukov F, Rihova L, Hajek R. Plasma cell leukemia: from biology to treatment. Eur J Haematol. 2015;95:16–26.
Rihova L, Vsianska P, Bezdekova R, Adam Z, Penka M, Jelinek T, et al. Identification of phenotype profile related to the extramedullary involvement in multiple myeloma relapse. Blood. 2016;128:5653–5653.
Deng S, Xu Y, An G, Sui W, Zou D, Zhao Y, et al. Features of extramedullary disease of multiple myeloma: high frequency of p53 deletion and poor survival: a retrospective single-center study of 834 cases. Clin Lymphoma Myeloma Leuk. 2015;15:286–91.
Royer B, Minvielle S, Diouf M, Roussel M, Karlin L, Hulin C, et al. Bortezomib, doxorubicin, cyclophosphamide, dexamethasone induction followed by stem cell transplantation for primary plasma cell leukemia: a prospective phase II study of the intergroupe francophone du myelome. J Clin Oncol. 2016;34:2125–32.
Avet-Loiseau H, Malard F, Campion L, Magrangeas F, Sebban C, Lioure B, et al. Translocation t(14;16) and multiple myeloma: is it really an independent prognostic factor? Blood. 2011;117:2009–11.
Neri A, Todoerti K, Lionetti M, Simeon V, Barbieri M, Nozza F, et al. Primary plasma cell leukemia 2.0: advances in biology and clinical management. Expert Rev Hematol. 2016;9:1063–73.
Usmani SZ, Nair B, Qu P, Hansen E, Zhang Q, Petty N, et al. Primary plasma cell leukemia: clinical and laboratory presentation, gene-expression profiling and clinical outcome with Total Therapy protocols. Leukemia. 2012;26:2398–405.
de Haart SJ, Willems SM, Mutis T, Koudijs MJ, van Blokland MT, Lokhorst HM, et al. Comparison of intramedullary myeloma and corresponding extramedullary soft tissue plasmacytomas using genetic mutational panel analyses. Blood Cancer J. 2016;6:e426.
Varga C, Xie W, Laubach J, Ghobrial IM, O’Donnell EK, Weinstock M, et al. Development of extramedullary myeloma in the era of novel agents: no evidence of increased risk with lenalidomide-bortezomib combinations. Br J Haematol. 2015;169:843–50.
Lopez-Anglada L, Gutierrez NC, Garcia JL, Mateos MV, Flores T, San Miguel JF. P53 deletion may drive the clinical evolution and treatment response in multiple myeloma. Eur J Haematol. 2010;84:359–61.
Chang H, Sloan S, Li D, Keith Stewart A. Multiple myeloma involving central nervous system: high frequency of chromosome 17p13.1 (p53) deletions. Br J Haematol. 2004;127:280–4.
Sheth N, Yeung J, Chang H. p53 nuclear accumulation is associated with extramedullary progression of multiple myeloma. Leuk Res. 2009;33:1357–60.
Billecke L, Murga Penas EM, May AM, Engelhardt M, Nagler A, Leiba M, et al. Cytogenetics of extramedullary manifestations in multiple myeloma. Br J Haematol. 2013;161:87–94.
Bezieau S, Devilder MC, Avet-Loiseau H, Mellerin MP, Puthier D, Pennarun E, et al. High incidence of N and K-Ras activating mutations in multiple myeloma and primary plasma cell leukemia at diagnosis. Hum Mutat. 2001;18:212–24.
Mulligan G, Lichter DI, Di Bacco A, Blakemore SJ, Berger A, Koenig E, et al. Mutation of NRAS but not KRAS significantly reduces myeloma sensitivity to single-agent bortezomib therapy. Blood. 2014;123:632–9.
Egan JB, Kortuem KM, Kurdoglu A, Izatt T, Aldrich J, Reiman R, et al. Extramedullary myeloma whole genome sequencing reveals novel mutations in Cereblon, proteasome subunit G2 and the glucocorticoid receptor in multi drug resistant disease. Br J Haematol. 2013;161:748–51.
Schinke CD, Ashby C, Wang Y, Tytarenko RG, Boyle E, Wardell C, et al. The mutational landscape of primary plasma cell leukemia. Blood. 2018;132:114–114.
Van Riet I, Vanderkerken K, de Greef C, Van Camp B. Homing behaviour of the malignant cell clone in multiple myeloma. Med Oncol. 1998;15:154–64.
Vanderkerken K, Van Camp B, De Greef C, Vande Broek I, Asosingh K, Van Riet I. Homing of the myeloma cell clone. Acta Oncol. 2000;39:771–6.
Moschetta M, Kawano Y, Sacco A, Belotti A, Ribolla R, Chiarini M, et al. Bone marrow stroma and vascular contributions to myeloma bone homing. Curr Osteoporos Rep. 2017;15:499–506.
Pellat-Deceunynck C, Barille S, Puthier D, Rapp MJ, Harousseau JL, Bataille R, et al. Adhesion molecules on human myeloma cells: significant changes in expression related to malignancy, tumor spreading, and immortalization. Cancer Res. 1995;55:3647–53.
Dahl IM, Rasmussen T, Kauric G, Husebekk A. Differential expression of CD56 and CD44 in the evolution of extramedullary myeloma. Br J Haematol. 2002;116:273–7.
Chang H, Bartlett ES, Patterson B, Chen CI, Yi QL. The absence of CD56 on malignant plasma cells in the cerebrospinal fluid is the hallmark of multiple myeloma involving central nervous system. Br J Haematol. 2005;129:539–41.
Liebisch P, Eppinger S, Schopflin C, Stehle G, Munzert G, Dohner H, et al. CD44v6, a target for novel antibody treatment approaches, is frequently expressed in multiple myeloma and associated with deletion of chromosome arm 13q. Haematologica. 2005;90:489–93.
van Driel M, Gunthert U, Stauder R, Joling P, Lokhorst HM, Bloem AC. CD44 isoforms distinguish between bone marrow plasma cells from normal individuals and patients with multiple myeloma at different stages of disease. Leukemia. 1998;12:1821–8.
Azab AK, Quang P, Azab F, Pitsillides C, Thompson B, Chonghaile T, et al. P-selectin glycoprotein ligand regulates the interaction of multiple myeloma cells with the bone marrow microenvironment. Blood. 2012;119:1468–78.
Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G, et al. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med. 2001;194:45–56.
Roccaro AM, Mishima Y, Sacco A, Moschetta M, Tai YT, Shi J, et al. CXCR4 regulates extra-medullary myeloma through epithelial-mesenchymal-transition-like transcriptional activation. Cell Rep. 2015;12:622–35.
Azab AK, Hu J, Quang P, Azab F, Pitsillides C, Awwad R, et al. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood. 2012;119:5782–94.
Wang SY, Hao HL, Deng K, Li Y, Cheng ZY, Lv C, et al. Expression levels of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and focal adhesion kinase in patients with multiple myeloma and their relationship to clinical stage and extramedullary infiltration. Leuk Lymphoma. 2012;53:1162–8.
Vacca A, Di Loreto M, Ribatti D, Di Stefano R, Gadaleta-Caldarola G, Iodice G, et al. Bone marrow of patients with active multiple myeloma: angiogenesis and plasma cell adhesion molecules LFA-1, VLA-4, LAM-1, and CD44. Am J Hematol. 1995;50:9–14.
Hedvat CV, Comenzo RL, Teruya-Feldstein J, Olshen AB, Ely SA, Osman K, et al. Insights into extramedullary tumour cell growth revealed by expression profiling of human plasmacytomas and multiple myeloma. Br J Haematol. 2003;122:728–44.
Montefusco V, Gay F, Spada S, De Paoli L, Di Raimondo F, Ribolla R, et al. Outcome of paraosseous extra-medullary disease in newly diagnosed multiple myeloma patients treated with new drugs. Haematologica. 2019; pii: haematol. 2019.219139. https://doi.org/10.3324/haematol.2019.219139.
Gagelmann N, Eikema DJ, Iacobelli S, Koster L, Nahi H, Stoppa AM, et al. Impact of extramedullary disease in patients with newly diagnosed multiple myeloma undergoing autologous stem cell transplantation: a study from the Chronic Malignancies Working Party of the EBMT. Haematologica. 2018;103:890–7.
Mangiacavalli S, Pompa A, Ferretti V, Klersy C, Cocito F, Varettoni M, et al. The possible role of burden of therapy on the risk of myeloma extramedullary spread. Ann Hematol. 2017;96:73–80.
Kumar L, Gogi R, Patel AK, Mookerjee A, Sahoo RK, Malik PS, et al. Multiple myeloma with extramedullary disease: impact of autologous stem cell transplantation on outcome. Bone Marrow Transpl. 2017;52:1473–5.
Weinstock M, Aljawai Y, Morgan EA, Laubach J, Gannon M, Roccaro AM, et al. Incidence and clinical features of extramedullary multiple myeloma in patients who underwent stem cell transplantation. Br J Haematol. 2015;169:851–8.
Short KD, Rajkumar SV, Larson D, Buadi F, Hayman S, Dispenzieri A, et al. Incidence of extramedullary disease in patients with multiple myeloma in the era of novel therapy, and the activity of pomalidomide on extramedullary myeloma. Leukemia. 2011;25:906–8.
Minnema MC, van de Donk NW, Zweegman S, Hegenbart U, Schonland S, Raymakers R, et al. Extramedullary relapses after allogeneic non-myeloablative stem cell transplantation in multiple myeloma patients do not negatively affect treatment outcome. Bone Marrow Transpl. 2008;41:779–84.
Perez-Simon JA, Sureda A, Fernandez-Aviles F, Sampol A, Cabrera JR, Caballero D, et al. Reduced-intensity conditioning allogeneic transplantation is associated with a high incidence of extramedullary relapses in multiple myeloma patients. Leukemia. 2006;20:542–5.
Raanani P, Shpilberg O, Ben-Bassat I. Extramedullary disease and targeted therapies for hematological malignancies-is the association real? Ann Oncol. 2007;18:7–12.
Ali R, Ozkalemkas F, Ozkan A, Ozkocaman V, Ozcelik T, Ozan U, et al. Bortezomib and extramedullary disease in multiple myeloma: the shine and dark side of the moon. Leuk Res. 2007;31:1153–5.
Zeiser R, Deschler B, Bertz H, Finke J, Engelhardt M. Extramedullary vs medullary relapse after autologous or allogeneic hematopoietic stem cell transplantation (HSCT) in multiple myeloma (MM) and its correlation to clinical outcome. Bone Marrow Transpl. 2004;34:1057–65.
Alegre A, Granda A, Martinez-Chamorro C, Diaz-Mediavilla J, Martinez R, Garcia-Larana J, et al. Different patterns of relapse after autologous peripheral blood stem cell transplantation in multiple myeloma: clinical results of 280 cases from the Spanish Registry. Haematologica. 2002;87:609–14.
Moreau P, Attal M, Caillot D, Macro M, Karlin L, Garderet L, et al. Prospective evaluation of magnetic resonance imaging and [(18)f]fluorodeoxyglucose positron emission tomography-computed tomography at diagnosis and before maintenance therapy in symptomatic patients with multiple myeloma included in the IFM/DFCI 2009 trial: results of the IMAJEM study. J Clin Oncol. 2017;35:2911–8.
Cavo M, Terpos E, Nanni C, Moreau P, Lentzsch S, Zweegman S, et al. Role of (18)F-FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: a consensus statement by the International Myeloma Working Group. Lancet Oncol. 2017;18:e206–e217.
Beksac M, Cengiz Seval G, Kanellias N, Coriu D, Rosinol L, Ozet G, et al. A real world multicenter retrospective study on extramedullary disease from Balkan Myeloma Study Group and Barcelona University: analysis of parameters that improve outcome. Haematologica. 2019; pii: haematol. 2019.219295. https://doi.org/10.3324/haematol.2019.219295. [Epub ahead of print].
Gonsalves WI, Rajkumar SV, Go RS, Dispenzieri A, Gupta V, Singh PP, et al. Trends in survival of patients with primary plasma cell leukemia: a population-based analysis. Blood. 2014;124:907–12.
Laura R, Cibeira MT, Uriburu C, Yantorno S, Salamero O, Blade J, et al. Bortezomib: an effective agent in extramedullary disease in multiple myeloma. Eur J Haematol. 2006;76:405–8.
Patriarca F, Prosdocimo S, Tomadini V, Vasciaveo A, Bruno B, Fanin R. Efficacy of bortezomib therapy for extramedullary relapse of myeloma after autologous and non-myeloablative allogeneic transplantation. Haematologica. 2005;90:278–9.
Ito C, Aisa Y, Mihara A, Nakazato T. Lenalidomide is effective for the treatment of bortezomib-resistant extramedullary disease in patients with multiple myeloma: report of 2 cases. Clin Lymphoma Myeloma Leuk. 2013;13:83–5.
Nakazato T, Mihara A, Ito C, Sanada Y, Aisa Y. Lenalidomide is active for extramedullary disease in refractory multiple myeloma. Ann Hematol. 2012;91:473–4.
Biagi JJ, Prince HM. Thalidomide is effective for extramedullary relapse of multiple myeloma post-allogeneic bone marrow transplantation. Br J Haematol. 2001;115:484–5.
Blade J, Perales M, Rosinol L, Tuset M, Montoto S, Esteve J, et al. Thalidomide in multiple myeloma: lack of response of soft-tissue plasmacytomas. Br J Haematol. 2001;113:422–4.
Rosinol L, Cibeira MT, Blade J, Esteve J, Aymerich M, Rozman M, et al. Extramedullary multiple myeloma escapes the effect of thalidomide. Haematologica. 2004;89:832–6.
Gagelmann N, Eikema DJ, Koster L, Caillot D, Pioltelli P, Lleonart JB, et al. Tandem autologous stem cell transplantation improves outcome in newly diagnosed multiple myeloma with extramedullary disease and high-risk cytogenetics: a study from the Chronic Malignancies Working Party of EBMT. Biol Blood Marrow Transplant. 2019;25:2134–42.
Muchtar E, Gatt ME, Rouvio O, Ganzel C, Chubar E, Suriu C, et al. Efficacy and safety of salvage therapy using Carfilzomib for relapsed or refractory multiple myeloma patients: a multicentre retrospective observational study. Br J Haematol. 2016;172:89–96.
Espanol I, Romera M, Gutierrez-Meca MD, Garcia MDC, Tejedor A, Martinez A, et al. Carfilzomib and dexamethasone for extramedullary myeloma with pleuropericardial involvement. Clin Case Rep. 2017;5:1258–60.
Chng WJ, Goldschmidt H, Dimopoulos MA, Moreau P, Joshua D, Palumbo A, et al. Carfilzomib-dexamethasone vs bortezomib-dexamethasone in relapsed or refractory multiple myeloma by cytogenetic risk in the phase 3 study ENDEAVOR. Leukemia. 2017;31:1368–74.
Dimopoulos M, Wang M, Maisnar V, Minarik J, Bensinger W, Mateos MV, et al. Response and progression-free survival according to planned treatment duration in patients with relapsed multiple myeloma treated with carfilzomib, lenalidomide, and dexamethasone (KRd) versus lenalidomide and dexamethasone (Rd) in the phase III ASPIRE study. J Hematol Oncol. 2018;11:49.
Usmani SZ, Weiss BM, Plesner T, Bahlis NJ, Belch A, Lonial S, et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood. 2016;128:37–44.
Pick M, Vainstein V, Goldschmidt N, Lavie D, Libster D, Gural A, et al. Daratumumab resistance is frequent in advanced-stage multiple myeloma patients irrespective of CD38 expression and is related to dismal prognosis. Eur J Haematol. 2018;100:494–501.
Musto P, Simeon V, Martorelli MC, Petrucci MT, Cascavilla N, Di Raimondo F, et al. Lenalidomide and low-dose dexamethasone for newly diagnosed primary plasma cell leukemia. Leukemia. 2014;28:222–5.
Mina R, Joseph NS, Kaufman JL, Gupta VA, Heffner LT, Hofmeister CC, et al. Survival outcomes of patients with primary plasma cell leukemia (pPCL) treated with novel agents. Cancer. 2019;125:416–23.
Gowda L, Shah M, Badar I, Bashir Q, Shah N, Patel K, et al. Primary plasma cell leukemia: autologous stem cell transplant in an era of novel induction drugs. Bone Marrow Transpl. 2019;54:1089–93.
Katodritou E, Terpos E, Delimpasi S, Kotsopoulou M, Michalis E, Vadikolia C, et al. Real-world data on prognosis and outcome of primary plasma cell leukemia in the era of novel agents: a multicenter national study by the Greek Myeloma Study Group. Blood Cancer J. 2018;8:31.
Jurczyszyn A, Radocha J, Davila J, Fiala MA, Gozzetti A, Grzasko N, et al. Prognostic indicators in primary plasma cell leukaemia: a multicentre retrospective study of 117 patients. Br J Haematol. 2018;180:831–9.
Ganzel C, Rouvio O, Avivi I, Magen H, Jarchowsky O, Herzog K, et al. Primary plasma cell leukemia in the era of novel agents for myeloma - a multicenter retrospective analysis of outcome. Leuk Res. 2018;68:9–14.
Jung SH, Lee JJ, Kim K, Suh C, Yoon DH, Min CK, et al. The role of frontline autologous stem cell transplantation for primary plasma cell leukemia: a retrospective multicenter study (KMM160). Oncotarget. 2017;8:79517–26.
Iriuchishima H, Ozaki S, Konishi J, Matsumoto M, Murayama K, Nakamura F, et al. Primary plasma cell leukemia in the era of novel agents: a multicenter study of the japanese society of myeloma. Acta Haematol. 2016;135:113–21.
Drake MB, Iacobelli S, van Biezen A, Morris C, Apperley JF, Niederwieser D, et al. Primary plasma cell leukemia and autologous stem cell transplantation. Haematologica. 2010;95:804–9.
Mahindra A, Kalaycio ME, Vela-Ojeda J, Vesole DH, Zhang MJ, Li P, et al. Hematopoietic cell transplantation for primary plasma cell leukemia: results from the center for international blood and marrow transplant research. Leukemia. 2012;26:1091–7.
Nooka AK, Kaufman JL, Muppidi S, Langston A, Heffner LT, Gleason C, et al. Consolidation and maintenance therapy with lenalidomide, bortezomib and dexamethasone (RVD) in high-risk myeloma patients. Leukemia. 2014;28:690–3.
Katodritou E, Terpos E, Kastritis E, Delimpasis S, Symeonidis AS, Repousis P, et al. Lack of survival improvement with novel anti-myeloma agents for patients with multiple myeloma and central nervous system involvement: the Greek Myeloma Study Group experience. Ann Hematol. 2015;94:2033–42.
Jurczyszyn A, Grzasko N, Gozzetti A, Czepiel J, Cerase A, Hungria V, et al. Central nervous system involvement by multiple myeloma: a multi-institutional retrospective study of 172 patients in daily clinical practice. Am J Hematol. 2016;91:575–80.
Gozzetti A, Cerase A, Lotti F, Rossi D, Palumbo A, Petrucci MT, et al. Extramedullary intracranial localization of multiple myeloma and treatment with novel agents: a retrospective survey of 50 patients. Cancer. 2012;118:1574–84.
Chen CI, Masih-Khan E, Jiang H, Rabea A, Cserti-Gazdewich C, Jimenez-Zepeda VH, et al. Central nervous system involvement with multiple myeloma: long term survival can be achieved with radiation, intrathecal chemotherapy, and immunomodulatory agents. Br J Haematol. 2013;162:483–8.
Lee D, Kalff A, Low M, Gangatharan S, Ho P, Bajel A, et al. Central nervous system multiple myeloma-potential roles for intrathecal therapy and measurement of cerebrospinal fluid light chains. Br J Haematol. 2013;162:371–5.
Abdallah AO, Atrash S, Shahid Z, Jameel M, Grazziutti M, Apewokin S, et al. Patterns of central nervous system involvement in relapsed and refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2014;14:211–4.
Paludo J, Painuly U, Kumar S, Gonsalves WI, Rajkumar V, Buadi F, et al. Myelomatous Involvement of the Central Nervous System. Clin Lymphoma Myeloma Leuk. 2016;16:644–54.
Mussetti A, Dalto S, Montefusco V. Effective treatment of pomalidomide in central nervous system myelomatosis. Leuk Lymphoma. 2013;54:864–6.
Vicari P, Ribas C, Sampaio M, Arantes AM, Yamamoto M, Filho JB, et al. Can thalidomide be effective to treat plasma cell leptomeningeal infiltration? Eur J Haematol. 2003;70:198–9.
Badros A, Singh Z, Dhakal B, Kwok Y, MacLaren A, Richardson P, et al. Marizomib for central nervous system-multiple myeloma. Br J Haematol. 2017;177:221–5.
Acknowledgements
This work is supported by the Carolinas Myeloma Research Fund, Heinemann Foundation of Charlotte, the Freedland Fund, the Leukemia Lymphoma Society and NCI Grant 5R01CA201634.
Author information
Authors and Affiliations
Contributions
SZU and MB conceived and designed the study. MB wrote the first draft of the manuscript with input from SZU and PMV. DMF, MB and SA prepared the figures. All authors were involved in researching data, discussion of content and critical revision of the paper and approval of the submitted manuscript.
Corresponding author
Ethics declarations
Conflict of interest
MB has received speaker’s fees from Amgen. DMF has no conflict of interest. SA has received consulting fees from Takeda, Amgen and Celgene, and speaker’s fees from Takeda and Celgene. PMV has received consulting fees from Amgen, BMS, Celgene, Janssen, Novartis, Oncopeptides, Takeda and TeneoBio. SZU has received consulting fees from Abbvie, Amgen, BMS, Celgene, EdoPharma, GSK, Janssen, Sanofi, Seattle Genetics, Skyline Dx, Takeda and TeneoBio, and research funding from Amgen, Array Biopharma, BMS, Celgene, Janssen, Pharmacyclics, Prothena, Sanofi, Seattle Genetics, Skyline Dx and Takeda.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhutani, M., Foureau, D.M., Atrash, S. et al. Extramedullary multiple myeloma. Leukemia 34, 1–20 (2020). https://doi.org/10.1038/s41375-019-0660-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0660-0
This article is cited by
-
Beyond the marrow: insights from comprehensive next-generation sequencing of extramedullary multiple myeloma tumors
Leukemia (2024)
-
1q21+ is associated with poor prognosis in newly diagnosed multiple myeloma patients with extramedullary disease: a retrospective study
Annals of Hematology (2024)
-
A novel two-step administration of XPO-1 inhibitor may enhance the effect of anti-BCMA CAR-T in relapsed/refractory extramedullary multiple myeloma
Journal of Translational Medicine (2023)
-
Comparison of clinical characteristics, treatment efficacy and survival in patients with newly diagnosed multiple myeloma with single- versus multi-site extramedullary invasion
Journal of Cancer Research and Clinical Oncology (2023)
-
Clinical relevance of high-risk cytogenetic abnormalities and the second revision of the International Staging System (R2-ISS) in patients with multiple myeloma in clinical practice
International Journal of Hematology (2023)