Abstract
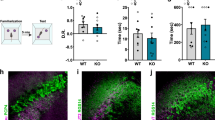
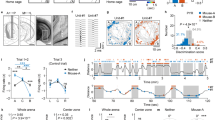
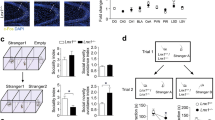
Social memory dysfunction is an especially devastating symptom of many neuropsychiatric disorders, which makes understanding the cellular and molecular processes that contribute to such abnormalities important. Evidence suggests that the hippocampus, particularly the CA2 region, plays an important role in social memory. We sought to identify potential mechanisms of social memory dysfunction in the hippocampus by investigating features of neurons, glia, and the extracellular matrix (ECM) of BTBR mice, an inbred mouse strain with deficient social memory. The CA2 is known to receive inputs from dentate gyrus adult-born granule cells (abGCs), neurons known to participate in social memory, so we examined this cell population and found fewer abGCs, as well as fewer axons from abGCs in the CA2 of BTBR mice compared to controls. We also found that BTBR mice had fewer pyramidal cell dendritic spines, in addition to fewer microglia and astrocytes, in the CA2 compared to controls. Along with diminished neuronal and glial elements, we found atypical perineuronal nets (PNNs), specialized ECM structures that regulate plasticity, in the CA2 of BTBR mice. By diminishing PNNs in the CA2 of BTBR mice to control levels, we observed a partial restoration of social memory. Our findings suggest that the CA2 region of BTBR mice exhibits multiple cellular and extracellular abnormalities and identify atypical PNNs as one mechanism producing social memory dysfunction, although the contribution of reduced abGC afferents, pyramidal cell dendritic spine, and glial cell numbers remains unexplored.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
22 April 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41380-022-01547-1
References
Williams DL, Goldstein G, Minshew NJ. Impaired memory for faces and social scenes in autism: clinical implications of memory dysfunction. Arch Clin Neuropsychol. 2005;20:1–15.
Porcelli S, Van Der Wee N, van der Werff S, Aghajani M, Glennon JC, van Heukelum S et al. Social brain, social dysfunction and social withdrawal. Neurosci Biobehav Rev. 2019;97:10–33.
Kurtz MM, Bronfeld M, Rose J. Cognitive and social cognitive predictors of change in objective versus subjective quality-of-life in rehabilitation for schizophrenia. Psychiatry Res. 2012;200:102–7.
García-Casal JA, Goñi-Imizcoz M, Perea-Bartolomé MV, Soto-Pérez F, Smith SJ, Calvo-Simal S, et al. The efficacy of emotion recognition rehabilitation for people with Alzheimer’s disease. J Alzheimers Dis. 2017;57:937–51.
Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10:47–56.
Montagrin A, Saiote C, Schiller D. The social hippocampus. Hippocampus. 2018;28:672–9.
Sheline YI, Mittler BL, Mintun MA. The hippocampus and depression. Eur Psychiatry. 2002;17:300–5.
Mak E, Gabel S, Su L, Williams GB, Arnold R, Passamonti L, et al. Multi-modal MRI investigation of volumetric and microstructural changes in the hippocampus and its subfields in mild cognitive impairment, Alzheimer’s disease, and dementia with Lewy bodies. Int Psychogeriatr. 2017;29:545–55.
Haukvik UK, Tamnes CK, Söderman E, Agartz I. Neuroimaging hippocampal subfields in schizophrenia and bipolar disorder: a systematic review and meta-analysis. J Psychiatr Res. 2018;104:217–26.
Hitti FL, Siegelbaum SA. The hippocampal CA2 region is essential for social memory. Nature. 2014;508:88–92.
Stevenson EL, Caldwell HK. Lesions to the CA2 region of the hippocampus impair social memory in mice. Eur J Neurosci. 2014;40:3294–301.
Smith AS, Williams Avram SK, Cymerblit-Sabba A, Song J, Young WS. Targeted activation of the hippocampal CA2 area strongly enhances social memory. Mol Psychiatry. 2016;21:1137–44.
Meira T, Leroy F, Buss EW, Oliva A, Park J, Siegelbaum SA. A hippocampal circuit linking dorsal CA2 to ventral CA1 critical for social memory dynamics. Nat Commun. 2018;9:4163.
Monteiro BMM, Moreira FA, Massensini AR, Moraes MFD, Pereira GS. Enriched environment increases neurogenesis and improves social memory persistence in socially isolated adult mice. Hippocampus. 2014;24:239–48.
Garrett L, Zhang J, Zimprich A, Niedermeier KM, Fuchs H, Gailus-Durner V, et al. Conditional reduction of adult born doublecortin-positive neurons reversibly impairs selective behaviors. Front Behav Neurosci. 2015;9:302.
Pereira-Caixeta AR, Guarnieri LO, Pena RR, Dias TL, Pereira GS. Neurogenesis inhibition prevents enriched environment to prolong and strengthen social recognition memory, but not to increase BdNF expression. Mol Neurobiol. 2017;54:3309–16.
Pereira-Caixeta AR, Guarnieri LO, Medeiros DC, Mendes EMAM, Ladeira LCD, Pereira MT, et al. Inhibiting constitutive neurogenesis compromises long-term social recognition memory. Neurobiol Learn Mem. 2018;155:92–103.
Cope EC, Waters RC, Diethorn EJ, Pagliai KA, Dias CG, Tsuda M, et al. Adult-born neurons in the hippocampus are essential for social memory maintenance. eNeuro. 2020;7:ENEURO.0182-20.2020.
Llorens-Martín M, Jurado-Arjona J, Avila J, Hernández F. Novel connection between newborn granule neurons and the hippocampal CA2 field. Exp Neurol. 2015;263:285–92.
Lensjø KK, Lepperød ME, Dick G, Hafting T, Fyhn M. Removal of perineuronal nets unlocks juvenile plasticity through network mechanisms of decreased inhibition and increased gamma activity. J Neurosci. 2017;37:1269–83.
Pantazopoulos H, Berretta S. In sickness and in health: perineuronal nets and synaptic plasticity in psychiatric disorders. Neural Plast. 2016;2016:9847696.
Sorg BA, Berretta S, Blacktop JM, Fawcett JW, Kitagawa H, Kwok JCF, et al. Casting a wide net: role of perineuronal nets in neural plasticity. J Neurosci. 2016;36:11459–68.
Yamada J, Ohgomori T, Jinno S. Perineuronal nets affect parvalbumin expression in GABAergic neurons of the mouse hippocampus. Eur J Neurosci. 2015;41:368–78.
Carstens KE, Phillips ML, Pozzo-Miller L, Weinberg RJ, Dudek SM. Perineuronal nets suppress plasticity of excitatory synapses on CA2 pyramidal neurons. J Neurosci. 2016;36:6312–20.
Domínguez S, Rey CC, Therreau L, Fanton A, Massotte D, Verret L, et al. Maturation of pnn and ERBb4 signaling in area CA2 during adolescence underlies the emergence of pv interneuron plasticity and social memory. Cell Rep. 2019;29:1099–112.e4.
Baig S, Wilcock GK, Love S. Loss of perineuronal net N-acetylgalactosamine in Alzheimer’s disease. Acta Neuropathol. 2005;110:393–401.
Yang M, Abrams DN, Zhang JY, Weber MD, Katz AM, Clarke AM, et al. Low sociability in BTBR T tf/J mice is independent of partner strain. Physiol Behav. 2012;107:649–62.
Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20.
Dityatev A, Rusakov DA. Molecular signals of plasticity at the tetrapartite synapse. Curr Opin Neurobiol. 2011;21:353–9.
York EM, Bernier LP, MacVicar BA. Microglial modulation of neuronal activity in the healthy brain. Dev Neurobiol. 2018;78:593–603.
Cope EC, Gould E. Adult neurogenesis, glia, and the extracellular matrix. Cell Stem Cell. 2019;24:690–705.
Torres L, Danver J, Ji K, Miyauchi JT, Chen D, Anderson ME, et al. Dynamic microglial modulation of spatial learning and social behavior. Brain Behav Immun. 2016;55:6–16.
Cope EC, LaMarca EA, Monari PK, Olson LB, Martinez S, Zych AD, et al. Microglia play an active role in obesity-associated cognitive decline. J Neurosci. 2018;38:8889–904.
McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–63.
Eichenbaum H. On the integration of space, time, and memory. Neuron. 2017;95:1007–18.
Seese RR, Maske AR, Lynch G, Gall CM. Long-term memory deficits are associated with elevated synaptic ERK1/2 activation and reversed by mGluR5 antagonism in an animal model of autism. Neuropsychopharmacology. 2014;39:1664–73.
Kohara K, Pignatelli M, Rivest AJ, Jung H-Y, Kitamura T, Suh J, et al. Cell type-specific genetic and optogenetic tools reveal hippocampal CA2 circuits. Nat Neurosci. 2014;17:269–79.
Stephenson DT, O’Neill SM, Narayan S, Tiwari A, Arnold E, Samaroo HD, et al. Histopathologic characterization of the BTBR mouse model of autistic-like behavior reveals selective changes in neurodevelopmental proteins and adult hippocampal neurogenesis. Mol Autism. 2011;2:7.
Tervo DGR, Hwang B-Y, Viswanathan S, Gaj T, Lavzin M, Ritola KD, et al. A designer AAV variant permits efficient retrograde access to projection neurons. Neuron. 2016;92:372–82.
Lein ES, Callaway EM, Albright TD, Gage FH. Redefining the boundaries of the hippocampal CA2 subfield in the mouse using gene expression and 3-dimensional reconstruction. J Comp Neurol. 2005;485:1–10.
San Antonio A, Liban K, Ikrar T, Tsyganovskiy E, Xu X. Distinct physiological and developmental properties of hippocampal CA2 subfield revealed by using anti-Purkinje cell protein 4 (PCP4) immunostaining. J Comp Neurol. 2014;522:1333–54.
Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–8.
Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705.
Chung W-S, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400.
Harada K, Kamiya T, Tsuboi T. Gliotransmitter release from astrocytes: functional, developmental, and pathological implications in the brain. Front Neurosci. 2015;9:499.
Beurdeley M, Spatazza J, Lee HHC, Sugiyama S, Bernard C, Di Nardo AA, et al. (2012) Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J Neurosci. 2012;32:9429–37.
Spatazza J, Lee HHC, Di Nardo AA, Tibaldi L, Joliot A, Hensch TK, et al. Choroid-plexus-derived Otx2 homeoprotein constrains adult cortical plasticity. Cell Rep. 2013;3:1815–23.
Dudek SM, Alexander GM, Farris S. Rediscovering area CA2: unique properties and functions. Nat Rev Neurosci. 2016;17:89–102.
Carstens KE, Dudek SM. Regulation of synaptic plasticity in hippocampal area CA2. Curr Opin Neurobiol. 2019;54:194–9.
McCann KE, Lustberg DJ, Shaughnessy EK, Carstens KE, Farris S, Alexander GM, et al. Novel role for mineralocorticoid receptors in control of a neuronal phenotype. Mol Psychiatry. 2019;19. https://doi.org/10.1038/s41380-019-0598-7.
Hayani H, Song I, Dityatev A. Increased excitability and reduced excitatory synaptic input into fast-spiking CA2 interneurons after enzymatic attenuation of extracellular matrix. Front Cell Neurosci. 2018;12:149.
Hylin MJ, Orsi SA, Moore AN, Dash PK. Disruption of the perineuronal net in the hippocampus or medial prefrontal cortex impairs fear conditioning. Learn Mem. 2013;20:267–73.
Lee HHC, Bernard C, Ye Z, Acampora D, Simeone A, Prochiantz A, et al. Genetic OTX2 mis-localization delays critical period plasticity across brain regions. Mol Psychiatry. 2017;22:680–8.
Foscarin S, Raha-Chowdhury R, Fawcett JW, Kwok JCF. Brain ageing changes proteoglycan sulfation, rendering perineuronal nets more inhibitory. Aging. 2017;9:1607–22.
Yang S, Sylvain G, Molinaro A, Naito-Matsui Y, Hilton S, Foscarin S, et al. Restoring the pattern of proteoglycan sulphation in perineuronal nets corrects age-related memory loss. bioRxiv. 2020. https://doi.org/10.1101/2020.01.03.894188.
Miyata S, Kitagawa H. Chondroitin 6-sulfation regulates perineuronal net formation by controlling the stability of aggrecan. Neural Plast. 2016;2016:1305801.
Yang S, Cacquevel M, Saksida LM, Bussey TJ, Schneider BL, Aebischer P, et al. Perineuronal net digestion with chondroitinase restores memory in mice with tau pathology. Exp Neurol. 2015;265:48–58.
Chafai M, Corbani M, Guillon G, Desarménien MG. Vasopressin inhibits LTP in the CA2 mouse hippocampal area. PLoS ONE. 2012;7:e49708.
Carstens KE, Lustberg DJ, Shaughnessy EK, McCann KE, Alexander GM, Dudek SM. MECP2 deletion prematurely restricts a novel window of hippocampal synaptic plasticity. Soc Neurosci. 2019. (abstract 187.07)
Sun ZY, Bozzelli PL, Caccavano A, Allen M, Balmuth J, Vicini S, et al. Disruption of perineuronal nets increases the frequency of sharp wave ripple events. Hippocampus. 2018;28:42–52.
Joo HR, Frank LM. The hippocampal sharp wave-ripple in memory retrieval for immediate use and consolidation. Nat Rev Neurosci. 2018;19:744–57.
Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S. Ventral CA1 neurons store social memory. Science. 2016;353:1536–41.
Daimon CM, Jasien JM, Wood WH 3rd, Zhang Y, Becker KG, Silverman JL, et al. Hippocampal transcriptomic and proteomic alterations in the BTBR mouse model of autism spectrum disorder. Front Physiol. 2015;6:324.
Provenzano G, Corradi Z, Monsorno K, Fedrizzi T, Ricceri L, Scattoni ML, et al. Comparative gene expression analysis of two mouse models of autism: transcriptome profiling of the BTBR and En2 (-/-) hippocampus. Front Neurosci. 2016;10:396.
Meyza KZ, Blanchard DC. The BTBR mouse model of idiopathic autism—current view on mechanisms. Neurosci Biobehav Rev. 2017;76:99–110.
del Zoppo GJ, Frankowski H, Gu Y-H, Osada T, Kanazawa M, Milner R, et al. Microglial cell activation is a source of metalloproteinase generation during hemorrhagic transformation. J Cereb Blood Flow Metab. 2012;32:919–32.
Zhang Z, Zhang Z, Lu H, Yang Q, Wu H, Wang J. Microglial polarization and inflammatory mediators after intracerebral hemorrhage. Mol Neurobiol. 2017;54:1874–86.
Pantazopoulos H, Gisabella B, Rexrode L, Benefield D, Yildiz E, Seltzer P, et al. Circadian rhythms of perineuronal net composition. eNeuro. 2020;7:ENEURO.0034-19.2020.
Nguyen PT, Dorman LC, Pan S, Vainchtein ID, Han RT, Nakao-Inoue H, et al. Microglial remodeling of the extracellular matrix promotes synapse plasticity. Cell. 2020;182:388–403.e15.
Jones-Davis DM, Yang M, Rider E, Osbun NC, da Gente GJ, Li J, et al. Quantitative trait loci for interhemispheric commissure development and social behaviors in the BTBR T+ tf/J mouse model of autism. PLoS ONE. 2013;8:e61829.
Wang M, He J, Zhou Y, Lv N, Zhao M, Wei H, et al. Integrated analysis of miRNA and mRNA expression profiles in the brains of BTBR mice. Int J Dev Neurosci. 2020;80:221–33.
Blanchard DC, Defensor EB, Meyza KZ, Pobbe RL, Pearson BL, Bolivar VJ, et al. BTBR T+tf/J mice: autism-relevant behaviors and reduced fractone-associated heparan sulfate. Neurosci Biobehav Rev. 2012;36:285–96.
Lang BT, Cregg JM, DePaul MA, Tran AP, Xu K, Dyck SM, et al. Modulation of the proteoglycan receptor PTPσ promotes recovery after spinal cord injury. Nature. 2015;518:404–8.
Pendleton JC, Shamblott MJ, Gary DS, Belegu V, Hurtado A, Malone ML, et al. Chondroitin sulfate proteoglycans inhibit oligodendrocyte myelination through PTPσ. Exp Neurol. 2013;247:113–21.
Acknowledgements
This work was supported by the National Institutes of Health, NIMH 1R01 MH118631-01 to EG and NIH Training Fellowship T32MH065214 to ECC. The authors thank Biorender for assistance with the figure schematics, Sahana Murthy and Brandy A. Briones for helpful comments on the project, and Carla G. Dias, Kristen A. Pagliai, and Amelia Windorski for assistance with histological analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In the original PDF of this article, pages 6-12 were inadvertently omitted.
Supplementary information
Rights and permissions
About this article
Cite this article
Cope, E.C., Zych, A.D., Katchur, N.J. et al. Atypical perineuronal nets in the CA2 region interfere with social memory in a mouse model of social dysfunction. Mol Psychiatry 27, 3520–3531 (2022). https://doi.org/10.1038/s41380-021-01174-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-021-01174-2
This article is cited by
-
Zinc-a2-Glycoprotein Acts as a Component of PNN to Protect Hippocampal Neurons from Apoptosis
Molecular Neurobiology (2024)
-
Maturation of nucleus accumbens synaptic transmission signals a critical period for the rescue of social deficits in a mouse model of autism spectrum disorder
Molecular Brain (2023)
-
Activation of the CA2-ventral CA1 pathway reverses social discrimination dysfunction in Shank3B knockout mice
Nature Communications (2023)
-
Rapid Eye Movement Sleep Consolidates Social Memory
Neuroscience Bulletin (2023)
-
Parvalbumin interneuron-derived tissue-type plasminogen activator shapes perineuronal net structure
BMC Biology (2022)