Abstract
The BRAFV600E mutation is a well-accepted poor prognostic factor in patients with metastatic colorectal cancer (mCRC), as it confers Ras-independent stimulation of the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway involved in proliferation, migration, angiogenesis and the suppression of apoptosis. Analysis of the potential predictive value of BRAF for treatment efficacy is inherently confounded by this known prognostic impact. Currently, approved therapeutic strategies for patients with BRAF-mutant (BRAF-mt) mCRC are suboptimal, and uncertainty exists regarding how to best treat these patients. Based on the available evidence, it is currently not possible to confirm the superiority of any available treatment options cited in European Society for Medical Oncology and National Comprehensive Cancer Network guidelines (that is, doublet or triplet chemotherapy regimens plus anti-vascular endothelial growth factor or anti-epidermal growth factor receptors), even if triplet chemotherapy plus bevacizumab is the most accepted standard regimen. In this review, we highlight still-emerging strategies that could be deployed to combat BRAF-mt mCRC, including triplet chemotherapy plus available biologic agents, rationally derived combinations of targeted agents and immunotherapy. While it is clear that the needs of patients with BRAF-mt mCRC are currently unmet, we are cautiously optimistic that the recently renewed research interest in these patients will yield clinically relevant insights and therapeutic strategies.
Similar content being viewed by others
Background
Despite significant progress in the treatment of colorectal cancer (CRC) over the past 15 years, the disease remains a leading cause of cancer-associated mortality worldwide.1 During the past decade, molecular testing in patients with metastatic CRC (mCRC) has become standard practice, and knowledge of RAS, BRAF and microsatellite instability (MSI) status is nowadays mandatory if we are to offer patients the best treatment and has contributed to the improved clinical outcome for patients with mCRC.2 Although it has been known since 2014 that mCRC caused by mutated RAS is resistant to anti-epidermal growth factor receptor (EGFR) therapy3,4 and since 2015 that the MSI phenotype is sensitive to immunotherapeutic agents,5,6 CRC patients with a mutation in BRAF are still awaiting a specific and tailored therapeutic approach. BRAF is a key downstream effector of RAS in the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) signal transduction pathway, which mainly influences cell proliferation, differentiation and apoptosis. BRAF is therefore considered to be an oncogenic driver in colorectal tumours,7 although the molecular, morphological, epidemiological and clinical characteristics of the serrated polyps initiated by BRAF differ from the polyps of the ‘classic’ adenoma–carcinoma sequence driven by mutations in the adenomatous polyposis coli (APC) gene.8
BRAF mutations, which are thought to be mutually exclusive of RAS mutations, arise in 5–10% of patients with mCRC.9 However, the prevalence of BRAF mutations might be underestimated because patients with these mutations are often ineligible for enrolment in clinical trials owing to their poor performance status and age. Indeed, the prevalence of BRAF mutations was recently reported to be as high as 21% in CRC patients in a Norwegian registry.10 The overwhelming majority (> 95%) of BRAF mutations in mCRC occur in codon 600, involving a T1799A transversion in exon 15, which results in the substitution of a valine amino acid for a glutamic acid (V600E mutation). Non-V600E BRAF mutations occur in ~2% of patients with mCRC and define a clinically distinct subtype with a better prognosis.11,12 Indeed, in a recent retrospective analysis of 2084 mCRC patients, overall survival (OS) was 39.4 months in patients with non-V600E BRAF mutations, whereas it was only 21 months in V600EBRAF-mutant patients. However, efficacy of anti-EGFR seemed limited in this cohort for non-V600E BRAF-mutant and RAS wild-type patients, and the predictive impact of these rare mutations remains unknown so far.13 BRAF-mt CRC used throughout this article will thus refer exclusively to the V600E mutation.
Gene-expression profiling studies have established that BRAFV600E-mutant BRAF-mt CRC is enriched in a molecularly and clinically distinct disease subtype, which is frequently associated with hypermethylation, MSI, limited chromosomal instability, consensus molecular subtype 1, a higher rate of recurrence in an adjuvant setting and poor survival outcomes in the metastatic setting.14,15
Numerous studies have confirmed the prognostic relevance of BRAF mutational status for both localised and metastatic colon cancers: patients with BRAF-mt CRC have impaired survival (Table 1) not only in the metastatic setting but also in non-metastatic disease as compared with patients with BRAF wild-type (BRAF-wt) CRC.11,16,17,18 Indeed, according to a meta-analysis of 11,321 patients, the risk of death was more than doubled in patients with BRAF-mt compared with those with BRAF-wt disease.19 Current therapeutic strategies, with doublet or triplet chemotherapies plus a targeted agent, for mCRC have achieved median OS exceeding 30 months in randomised phase 3 clinical trials involving patients with RAS wild-type mCRC,20,21 and 25 months in mCRC patients not selected for their RAS status.22 A recent meta-analysis restricted to patients with KRAS-wt mCRC reported significantly impaired survival in patients with BRAF-mt/KRAS wt disease, with a median OS of 10.8 months.23
Given this poor outcome in patients with BRAF-mt mCRC, the optimisation of therapy is an important goal. In this review article, we summarise current treatment options for patients with BRAF-mt mCRC, as well as emerging strategies that, taken together, show the continued need for additional dedicated studies in these patients.
The BRAF pathway
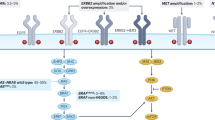
The RAS/MAPK pathway, together with the PI3K (phosphatidylinositol 3-kinase)/AKT pathway, constitutes one of the best-known signal transmission pathways resulting, after a cascade of successive phosphorylations, in the transcription of genes involved in cancer development. The MAPK/ERK signalling cascade conveys mitogenic and other stimulatory signals from receptors, such as EGFR, on the cell membrane to the nucleus. Activation of the RAF family of serine/threonine kinases proteins by a Ras small guanidine triphosphatase (GTPase) downstream of cell–surface receptors leads to the phosphorylation and activation of MAPK and ERK kinase (MEK)1/2 proteins, which subsequently phosphorylate and activate ERK1/2 proteins. Upon activation, ERK proteins phosphorylate a variety of substrates, including multiple transcription factors, and regulate several key cellular activities, such as proliferation, migration, angiogenesis and the suppression of apoptosis (Fig. 1a). The RAF family also includes ARAF (also known as ARAF1) and CRAF (also known as RAF1), but BRAF has the strongest basal kinase activity and is the most potent activator of MEK/ERK proteins.
The BRAF pathway. a Activated BRAF-mutated protein leads to phosphorylation and activation of mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) kinase (MEK)1/2 proteins, which subsequently phosphorylate and activate ERK1/2 proteins. After activation, ERK proteins phosphorylate a variety of substrates, including multiple transcription factors and regulate several key cellular activities, such as proliferation, differentiation and angiogenesis, to promote tumour growth. b Inhibition of BRAF suppresses the ERK-mediated negative feedback of the epidermal growth factor receptor (EGFR), resulting in EGFR activation, formation of RAF protein dimers and CRAF-mediated reactivation of the MAPK signalling pathway. c Preclinical studies have shown efficacy with combination drugs targeting BRAF (BRAF inhibitor), MEK (MEK inhibitor) and EGFR (anti-EGFR monoclonal antibody); this triplet combination might be an interesting therapeutic approach in patients with BRAF-mutated mCRC. d Crosstalk between the RAS/BRAF/MEK/ERK and the PI3K/AKT/mammalian target of rapamycin (mTor) signalling pathways after BRAF inhibition could play a determinant role in cell survival. Combining BRAF, EGFR and PI3K inhibitors could constitute another interesting therapeutic approach in patients with BRAF-mutated mCRC
Aberrant signalling or inappropriate activation of the MAPK/ERK signalling pathway is involved in many human malignancies.24 Among solid tumours, the highest prevalence of activating somatic missense BRAF mutations, with the V600E substitution accounting for ~80% of mutations, occurs in malignant melanomas (60–70%); mutations occur at a lower frequency in other human cancers, such as papillary and anaplastic thyroid carcinomas (40–50%), ovarian (30%) and CRCs (10–20%). All mutations in BRAF confer increased kinase activity compared with the wild-type protein, and thereby stimulate MAPK/ERK activity in a Ras-independent manner.7
Finally, BRAF-mt patients should not be considered as having a unique biology. In fact, Barras et al.25 have even recently described, from a series of 218 BRAF-mt patients with colon cancer, two distinct subtypes of patient, independent of their gender, primary tumour location, mismatch repair (MMR) status and PI3K status. The BM1 subtype, representing one-third of patients, is associated with the strong activation of AKT/mammalian target of rapamycin (mTOR), KRAS, 4EBP1 and epithelial–mesenchymal transition features, whereas BM2, representing the remaining two-thirds of BRAF-mt patients, displays deregulation of the cell cycle, with high levels of cyclin-dependent kinase (CDK)1 and low cyclin D1.
BRAF inhibitors have proven clinical activity in BRAF-mutant patients in other tumour locations, such as melanoma, but resistance emerges frequently due to a multitude of escape mechanisms, thereby necessitating combination treatment (Fig. 1b). For instance, in vitro studies have suggested that BRAF inhibition suppresses ERK-mediated negative feedback on EGFR activity, resulting in EGFR activation, the formation of RAF protein dimers and CRAF-mediated reactivation of the ERK/MAPK signalling pathway. Thus, EGFR signalling seems to play a critical role in bypassing BRAF inhibition and mediating therapeutic resistance.26,27 This may explain partly the insufficient efficacy of anti-BRAF monotherapy since BRAF inhibition induces a simultaneous overexpression of EGFR receptor leading on the one hand to the adaptive feedback reactivation of MAPK signalling via CRAF and on the other hand to the activation of the PI3K/AKT pathway, which also depends on the EGFR signal. To overcome these resistance mechanisms, blocking both BRAF, EGFR and MEK (Fig. 1c) and combining BRAF and PI3K inhibitors make sense (Fig. 1d).
Current systemic treatments for BRAF-mt mCRC
Current standard first-line chemotherapy for mCRC patients involves the combination of a fluoropyrimidine and either irinotecan or oxaliplatin. Standard chemotherapy has been evaluated in a retrospective cohort of 127 BRAF-mt mCRC patients, and has shown very poor outcomes in terms of progression-free survival (PFS) for the first three lines of chemotherapy (median PFS of 6.3, 2.5 and 2.6 months, respectively). The choice of systemic therapy used (oxaliplatin-based or irinotecan-based regimen) did not significantly affect PFS in first-line treatment (6.4 versus 5.4 months, P = 0.99).28
A more aggressive strategy, involving combination of doublet with the EGFR inhibitor, cetuximab, or triplet with bevacizumab, which inhibits vascular endothelial growth factor (VEGF), might be of interest in mCRC patients with BRAF-mt tumours, as suggested by some clinical data, although these data are based on small subgroups.17,29,30,31,32 Indeed, in patients with KRAS-wt/BRAF-mt mCRC included in the CRYSTAL randomised trial comparing FOLFIRI (folinic acid, fluorouracil and irinotecan) alone or combined with cetuximab, a trend to improvement in both PFS (median, 8.0 versus 5.6 months; HR, 0.93; P = 0.87) and OS (median, 14.1 versus 10.3 months; HR, 0.91; P = 0.74) was observed in favour of the anti-EGFR monoclonal antibody-based treatment.17 In a 2010 study by Masi et al.,29 patients with wild-type and mutated BRAF CRC tumours had similar median PFS and OS when the treatment was based on the triplet FOLFOXIRI (folinic acid, fluorouracil, oxaliplatin and irinotecan) with bevacizumab, suggesting that this aggressive therapeutic strategy could also lead to the loss of the negative prognostic impact of BRAF mutation. Loupakis et al.30 conducted one of the first phase 2 studies conceived to explore an intensified regimen with FOLFOXIRI plus bevacizumab specifically in BRAF-mt patients (15 patients) and showed interesting results (median OS, 19 months; median PFS, 7.5 months). Finally, in a randomised phase 3 trial comparing bevacizumab plus FOLFIRI to bevacizumab plus FOLFOXIRI in mCRC patients, the subgroup of BRAF-mt patients appeared to benefit from the addition of oxaliplatin in terms of OS [19 versus 10.7 months; HR, 0.54 (95% CI, 0.24–1.20)], and PFS [7.5 versus 5.5 months; HR, 0.57 (95% CI, 0.27–1.23)], although this survival benefit did not reach statistical significance.22,31
These results led to the recommendation of this upfront, aggressive schedule in patients with BRAF-mt mCRC in most recent guidelines.2,33,34 However, it is important to emphasise that this ‘standard treatment’ is based on the observation of fewer than 100 patients in three studies. However, even if the level of evidence remains weak, this strategy is well accepted because it offers an aggressive upfront treatment, including all major chemotherapeutic agents for mCRC and a targeted therapy, with a manageable toxicity profile, to treat patients with a particularly aggressive disease who are rarely able to receive a second-line treatment.
Efficacy of registered targeted agents
Anti-angiogenic agents
Although it has been shown that the MAPK signalling cascade can increase VEGF expression and that BRAF mutation might also modulate tumour response to anti-angiogenic treatments,35 the value of bevacizumab in BRAF-mt patients has not yet been clinically demonstrated. In fact, in the previously reported results, although the addition of oxaliplatin to FOLFIRI plus bevacizumab treatment seemed beneficial over FOLFIRI plus bevacizumab treatment, the added value of the anti-angiogenic agent has not been shown.31 However, even if no randomised data evaluating the influence of adding bevacizumab to standard chemotherapy (i.e., FOLFIRI or FOLFOX) are available from patients with BRAF-mt mCRC, the addition of bevacizumab to first-line IFL [bolus irinotecan, fluorouracil and leucovorin (folinic acid)] or capecitabine has shown a numerical improvement in survival outcomes in patients with BRAF-mt mCRC in post-hoc analyses of the AVF2107g35 and AGITG MAX36 trials. In addition, the results of the VELOUR trial biomarker analysis37 have recently been reported. The corresponding clinical trial randomised aflibercept [a fusion protein that binds circulating VEGF-A, VEGF-B and placental growth factor (PlGF)] versus placebo, in combination with FOLFIRI chemotherapy, in second-line treatment. For the biomarker analysis, 482 samples were collected from 1226 randomised patients (39% of the patients) with mCRC who progressed after oxaliplatin-based first-line chemotherapy. The results showed that the BRAF-mutated population (n = 36, 7.5%) benefitted more from addition of aflibercept [OS HR, 0.42, (95% CI, 0.16–1.09)] than did the BRAF-wt population, but the difference was not significant [HR, 0.49 (95% CI, 0.22–1.09), P = 0.08], probably due to the small series of patients. Similar results were reported with the RAISE trial biomarker analysis38 using FOLFIRI in second-line treatment with another anti-angiogenic agent, ramucirumab, that targets VEGFR2. Although these post-hoc analyses of randomised trials suggest that anti-angiogenic agents might be of interest in BRAF-mt mCRC patients, prospective trials comparing an aggressive chemotherapy alone or in combination with an anti-angiogenic therapy are still awaited.
Anti-EGFR agents
Concerning anti-EGFR agents, current data and publications are confusing. Nevertheless, it seems quite obvious that anti-EGFR monoclonal antibodies (panitumumab and cetuximab) provide no benefit for BRAF-mt mCRC patients when these therapies are used as single agents in patients heavily pre-treated with chemotherapy.39 Similarly, in second-line treatment, two studies evaluating the addition of anti-EGFR to FOLFIRI have reported the same results, with no clinical benefit to BRAF-mt mCRC patients.40,41 The PICCOLO trial even reported a deleterious effect, in terms of OS [HR, 1.84 (95% CI, 1.10–3.08), P = 0.029], of adding panitumumab to irinotecan treatment in patients with BRAF-mt tumours.41
The results of first-line treatment using the combination of chemotherapy plus anti-EGFR agents are less clear. The pooled analysis data of CRYSTAL and OPUS randomised studies evaluating the addition of cetuximab to first-line FOLFIRI or FOLFOX chemotherapy in KRAS-wt mCRC patients have shown an improvement of objective response rate (ORR), PFS and OS in the subgroup of BRAF-mt mCRC patients.32 The authors concluded that the BRAF mutation does not appear to be a predictive biomarker of resistance to anti-EGFR therapy in this setting, only a marker of poor prognosis. Similarly, the addition of panitumumab to FOLFOX first-line chemotherapy was associated with a numerical improvement of efficacy outcomes in the KRAS-wt/BRAF-mt subgroup.4
Two meta-analyses have been performed on the results from phase 2 and 3 clinical trials using cetuximab or panitumumab alone or combined with chemotherapy in first-, second- or beyond-second-line treatment. The first meta-analysis reported that anti-EGFR agents did not significantly improve survival for BRAF-mt mCRC patients [nor PFS (HR, 0.88; P = 0.33) or OS (HR, 0.91; P = 0.63)] compared with standard chemotherapy or best supportive care.42 The second meta-analysis showed no significant interaction between anti-EGFR treatment and BRAF status for PFS and OS; the authors concluded that the BRAF mutation could not actually be considered as a negative predictive biomarker for anti-EGFR monoclonal antibodies in mCRC—that is, the presence of mutated BRAF should not preclude patients from receiving anti-EGFR therapy—and that further data are required to clarify this observation.43 Both these meta-analyses are subject to many limitations, and overall cannot guide our practice. First, not all available studies were included in these two meta-analyses; second, several lines of treatment with different populations and expected survival were mixed; third, negative trials for anti-EGFR agents with irrelevant backbone chemotherapeutic regimens (such as capecitabine plus oxaliplatin) were included; fourth, control arms mixed various chemotherapy regimens or even best supportive care; and fifth, both panitumumab and cetuximab trials were mixed although they might give different results in BRAF-mt patients. All these points are likely to present significant confounding factors when evaluating BRAF-mt mCRC patients.
Recently, a randomised phase 2 trial has evaluated the effect of adding panitumumab to triplet chemotherapy in first-line RAS wild-type mCRC patients. The addition of anti-EGFR agents to FOLFOXIRI improved the response rate in the whole study population of 96 patients (ORR, 85.7% versus 60.6%, P = 0.0096), without improving PFS (OS data not available). In a subgroup of BRAF-mt patients, the ORR also improved impressively (71% versus 22%), even though statistical significance was not reached, probably due to the limited number of BRAF-mt patients (n = 16).44
Although anti-EGFR agents do not confer any benefit to pre-treated BRAF-mt mCRC patients, these results suggest that they might be of value in the first-line treatment of such patients, especially if the goal of the treatment is tumour shrinkage. However, as stated above for anti-angiogenic therapies, trials comparing an aggressive chemotherapy ± an anti-EGFR therapy dedicated to BRAF-mt mCRC patients are still awaited. Finally, the FIRE-3 trial has compared FOLFIRI plus bevacizumab with FOLFIRI plus cetuximab in the first-line treatment of RAS wt mCRC patients. For the 48 (n = 14%) BRAF-mt patients identified in this trial, the ORR was higher in the cetuximab arm than in the bevacizumab arm (52% versus 40%), while no statistical differences were observed for PFS (HR, 0.84, P = 0.56) and OS (HR, 0.79, P = 0.45),45 suggesting that EGFR and VEGF inhibitors have equivalent therapeutic efficacy in BRAF-mt mCRC patients, except for response rate that favours anti-EGFRs.
Targeting BRAF
BRAF mutations are found in many cancers and are particularly common in melanoma. In patients with V600E BRAF-mt metastatic melanoma, vemurafenib, a tyrosine kinase inhibitor specific to the ATP-binding domain of BRAF V600E, significantly improves both OS and PFS compared with dacarbazine, and facilitates response rates of 48% (versus only 5% with dacarbazine).46 However, the beneficial effect of BRAF-inhibitor monotherapy, using either vemurafenib or encorafenib, another ATP-competitive kinase inhibitor, seems much more limited in patients with BRAF-mt mCRC, with fewer than 10% of responders and PFS of 2.1–4.3 months.47,48,49,50 Based on these data, BRAF inhibitors alone seem to have insufficient clinical activity in patients with BRAF -mt CRC.
Combining BRAF inhibitors and anti-EGFR agents
Preclinical studies conducted on BRAF-mt mCRC cell lines have shown that BRAF inhibition leads to the rapid feedback activation of EGFR, which could explain the persistence of tumour proliferation despite BRAF inhibition, as shown on Fig. 1b.51 Lower levels of EGFR expression by cancerous melanoma cells compared with CRC cells might explain the observed differences between melanoma and CRC in terms of response rates to BRAF-inhibitor monotherapy. Accordingly, the addition of cetuximab to encorafenib had a synergistic anti-proliferative effect in a human xenograft model of BRAF-mt CRC.27 In a pilot trial of 15 patients with BRAF-mt mCRC, the combination of vemurafenib and panitumumab induced modest anti-tumour activity. Tumour regression was seen in 10 of 12 patients, with partial responses in two patients (100 and 64% regression lasting 40 and 24 weeks, respectively) and stable disease lasting over 6 months in two others.52 In a basket trial, only one response was observed in the group of patients with mCRC who received vemurafenib combined with cetuximab, although tumour regression was observed in several other patients, albeit without fulfilling the RECIST 1.1 partial response criteria. Median PFS and OS values for these patients were 3.7 (95% CI, 1.8– 5.1) and 7.1 months (95% CI, 4.4 to not reached), respectively.50
More interestingly, when vemurafenib at different doses was combined with cetuximab and irinotecan in 17 BRAF-mt CRC patients in a phase 1b study, partial responses were observed in 35% of patients, with a median PFS of 7.7 months.53 The SWOG S1406 study then randomised 99 patients with BRAF-mt mCRC pre-treated with one or two lines of systemic chemotherapy to two arms of irinotecan plus cetuximab plus vemurafenib, with PFS as the primary objective.54 Median PFS was 4.4 months with the triplet therapy versus 2.0 months in patients treated with the doublet cetuximab plus irinotecan (HR, 0.42; P = 0.0002). Response rate and disease control rate (DCR) were also significantly higher for patients receiving the triplet drug combination (ORR, 16% versus 4%, P = 0.09; and DCR, 67% versus 22%, P < 0.001, respectively). Side effects were more common in the triplet arm, comprising mainly neutropenia, anaemia, nausea and arthralgia, and led to treatment discontinuation in 18% of cases. The subgroup analyses of this study should also provide more data about the efficacy of this triplet approach, especially in BRAF-mt MSI patients. Despite the limited number of patients included in the above-mentioned studies, this new strategy of double EGFR–BRAF inhibition shows undeniable signs of activity, and could represent a promising therapeutic option for BRAF-mt mCRC patients in the future.
Combining BRAF inhibitors, anti-EGFRs and PI3K/AKT or MEK inhibitors
In preclinical studies, CRC cell lines also show high levels of PI3K/AKT pathway activation, which might contribute to resistance to BRAF-targeted monotherapy, as shown in Fig. 1d.55 In fact, the activation of this alternative pathway has already been described as a classical resistance mechanism to BRAF/RAS/MAPK pathway blockade. In BRAF-mt mCRC patients, a phase 1b trial has evaluated the therapeutic effect of encorafenib with cetuximab (doublet) ± alpelisib (an α-specific PI3K inhibitor) (triplet) in 28 patients.56 Best ORR and PFS were, respectively, 23.1% and 3.7 months (95% CI, 2.8–10.6) in the dual arm versus 32.1% and 4.3 months (95% CI, 4.1–5.4) in patients treated with the triplet, which seemed relatively well tolerated. The most common treatment-related grade 3/4 effects were fatigue and hypophosphataemia (8% each) in patients treated with the doublet, and hyperglycaemia (11%) and increased lipase (7%) in the triplet arm.
Combination strategies involving both MEK and BRAF inhibitors together with anti-EGFRs also significantly improved PFS in previously untreated melanoma patients.57 The combination of the BRAF inhibitor, dabrafenib, with panitumumab and the MEK inhibitor trametinib has also been tested with interesting results (ORR 26%, median PFS 4.1 months), with the limitation of significant skin toxicities.57 The combination of the BRAF inhibitor, dabrafenib, with panitumumab and the MEK inhibitor trametinib (ORR, 26%; median PFS, 4.1 months), with the limitation of significant skin toxicities.58
Thus, combining inhibition of EGFR and MAPK pathways with BRAF-targeted therapies together with a MEK inhibitor or with an action on the PI3K/mTOR alternative pathway using a PI3K inhibitor might be promising options to improve outcomes of BRAF-mt mCRC patients, and several trials are currently underway (Table 2). An open-label large phase 1 study has recently evaluated the triple combination of BRAF/MEK/EGFR inhibitors (as shown in Fig. 1c) in 142 patients with BRAF-mt CRC, and shows promising results (confirmed response rates of 21%) with an acceptable safety profile, with mostly dermatological toxicity.51 However, further randomised studies are required for a number of reasons: first, to find the most effective combination; second, to improve the tolerability of these combination therapies; and third, to compare them with standard chemotherapeutic regimens.
Further biomarker analyses will also be required to clarify the link between the genetic characteristics of the tumour and the response to treatment. Notably, combination strategies involving WNT pathway inhibitors in patients with BRAF-mt mCRC may be justified in the future by the observation of the association between WNT5A promoter methylation and BRAFV600E mutation in CRC patients.59
Immunotherapies
Targeting the immune system is a promising therapeutic option to improve the survival of some cancer patients, as shown in recent clinical trials involving immune checkpoint inhibitors in several tumour locations.60,61 However, studies evaluating immunotherapy in CRC patients, especially those using antibodies against programmed cell death protein 1 (PD1), have yielded disappointing results, with the exception of the subgroup of MSI patients, which is characterised by a strong immune infiltrate.62 Several studies have highlighted the overlap between the presence of BRAFV600E mutations and MSI in CRC tumours.7,15,63,64 Indeed, BRAF-mt tumours are associated with the CpG island methylator phenotype (CIMP), which can lead to the inactivation of the MLH1 promoter, resulting in an MMR deficiency.63 In a pooled analysis of the CAIRO, CAIRO2, COIN and FOCUS studies involving primary tumours from 3063 patients, BRAF mutations were observed in 34.6% of patients with MSI tumours, whereas among BRAF-mt tumours 21.2% showed MSI.65 Higher correlation levels were found in a cohort study of 1253 patients, in which 52% of MSI tumours also had BRAF mutations, while 55% of the BRAF-mt tumours showed MSI.66
Given the encouraging results obtained in the MSI subgroup of CRC patients treated with PD1 inhibitors, it seems that there is an undeniable value in evaluating checkpoint inhibitors in the specific subgroup of MSI BRAF-mt patients. In addition, a positive correlation between the expression of programmed death ligand-1 (PD-L1) and the presence of mutated BRAFV600E has been shown in BRAF-mt tumours, with higher levels of CD8+ tumour-infiltrating lymphocytes observed in BRAF-mt colorectal tumours,67 suggesting that BRAF-mt mCRC patients might benefit from immunotherapy.
In the CheckMate 142 trial, nivolumab, a checkpoint inhibitor targeting PD1, was tested in 74 pre-treated MSI mCRC patients, 12 (16%) of whom had BRAF-mt tumours. ORR and DCR for 12 weeks and more were, respectively, 31 and 69% versus 25 and 75% in BRAF-mt patients.68 Higher response rates were observed in the cohort of patients treated with nivolumab plus ipilimumab (a CTLA-4 inhibitor) (n = 119) in the same study, with an ORR of 55% and a DCR of 80% (median follow-up of 13.4 months). Interestingly, in BRAF-mt patients (n = 29), the ORR was not lower (55%) and the DCR > 12 weeks was 79%.69
Considering these results, it seems that immune checkpoint blockade may be more effective than BRAF-targeted therapies for BRAF-mt MSI mCRC patients. However, based on preclinical data that have shown an increase in the levels of both tumour antigens and the expression of major histocompatibility complex (MHC) molecules in patients treated with vemurafenib, combinations of immune checkpoint blockers and BRAF-targeted therapies are currently being tested in melanoma patients.70 This approach will need to be tested in the future for MSI–BRAF-mt mCRC patients.
Ongoing studies
The BEACON study is the first multicentre, randomised, open-label, phase 3 three-arm study dedicated to BRAF-mt mCRC. The study compares, in mCRC patients pre-treated by one or two lines of treatment, the triplet encorafenib plus binimetinib (MEK inhibitor) plus cetuximab versus the doublet encorafenib plus cetuximab versus irinotecan plus cetuximab or FOLFIRI plus cetuximab (control arm) with OS as the primary objective in patients with BRAF-mt mCRC. After a median duration of follow-up of 18.2 months, results based on 29 patients with a BRAFV600E mutation treated for a median duration of 5.6 months were promising, with an ORR of 48% (three complete and 11 partial responses), a median PFS of 8.0 months and a median OS of 15.3 months. Analysis of the safety lead-in cohort of the BEACON trial suggests an acceptable and manageable safety profile for patients receiving the encorafenib, binmetinib, and cetuximab combination. Dose-limiting toxicities occurred in five patients (including serous retinopathy and reversible decreased left ventricular ejection fraction) and were related to cetuximab-related infusion reactions for two of them.71 A very recent press release mentioned that the interim analysis of this study showed that the doublet (cetuximab + encorafenib) and the triplet (cetuximab + encorafenib + binimetinib) increased ORRs from 1.9% in the control arm to 20.4 and 26.1% in the experimental arms, respectively. OS was also improved in the two experimental arms with HR of 0.52 (95% CI, 0.39–0.70; P < 0.0001) and 0.60 (95% CI, 0.45–0.79; P = 0.0003). Full results of this interim analysis will be communicated in the forthcoming ESMO meetings.
Another phase 3 randomised trial designed to investigate FOLFOXIRI plus cetuximab or FOLFOXIRI plus bevacizumab as first-line treatment in BRAF-mt mCRC patients is currently underway, with a main objective of ORR (FIRE-4.5/AIO KRK-0116). Further phase 1/2 studies testing the efficacy and safety of combination therapies involving other BRAF inhibitors, PI3K, WNT and MEK inhibitors are currently ongoing and are summarised in Table 3.
Conclusion
The BRAFV600E mutation is a major negative prognostic marker and is associated with resistance to standard chemotherapeutic regimens in mCRC patients, which justifies a personalised therapeutic approach in BRAF-mt mCRC patients. Although the best treatment has not yet been identified, an aggressive strategy involving triplet chemotherapy and a targeted therapy is currently the standard of care for fit patients. BRAF-targeted therapies have shown insufficient efficacy when used alone, but their combination with other targeted therapies such as anti-EGFRs, MEK inhibitors or PI3K inhibitors seems promising. Checkpoint inhibitors might also find their place in BRAF-mt mCRC patients with MSI, given the overlap between the BRAF mutation and the MSI phenotype. Finally, the place of each of the therapeutic combinations described and the way to sequence these new options remains an open question today. Further investigations are therefore justified, hence the need to promote the enrolment of BRAF-mt mCRC patients in clinical trials.
References
Ferlay, J., Soerjomataram, I., Dikshit, R., Eser, S., Mathers, C., Rebelo, M. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386 (2015).
Van Cutsem, E., Cervantes, A., Adam, R., Sobrero, A., Van Krieken, J. H., Aderka, D. et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 27, 1386–1422 (2016).
Lièvre, A., Bachet, J.-B., Boige, V., Cayre, A., Le Corre, D., Buc, E. et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J. Clin. Oncol. 26, 374–379 (2008).
Douillard, J.-Y., Oliner, K. S., Siena, S., Tabernero, J., Burkes, R., Barugel, M. et al. Panitumumab–FOLFOX4 treatment and RAS mutations in colorectal cancer. N. Engl. J. Med. 369, 1023–1034 (2013).
Le, D. T., Uram, J. N., Wang, H., Bartlett, B. R., Kemberling, H., Eyring, A. D. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
Andre, T., Lonardi, S., Wong, M., Lenz, H.-J., Gelsomino, F., Aglietta, M. et al. Nivolumab + ipilimumab combination in patients with DNA mismatch repair-deficient/microsatellite instability-high (dMMR/MSI-H) metastatic colorectal cancer (mCRC): first report of the full cohort from CheckMate-142. J. Clin. Oncol. 36(4 Suppl), 553–553 (2018).
Davies, H., Bignell, G. R., Cox, C., Stephens, P., Edkins, S., Clegg, S. et al. Mutations of the BRAF gene in human cancer. Nature 417, 949 (2002).
Rad, R., Cadiñanos, J., Rad, L., Varela, I., Strong, A., Kriegl, L. et al. A genetic progression model of BrafV600E-induced intestinal tumorigenesis reveals targets for therapeutic intervention. Cancer Cell 24, 15–29 (2013).
De Roock, W., Claes, B., Bernasconi, D., De Schutter, J., Biesmans, B., Fountzilas, G. et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 11, 753–762 (2010).
Sorbye, H., Dragomir, A., Sundström, M., Pfeiffer, P., Thunberg, U., Bergfors, M. et al. High BRAF mutation frequency and marked survival differences in subgroups according to KRAS/BRAF mutation status and tumor tissue availability in a prospective population-based metastatic colorectal cancer cohort. PLoS ONE 10, e0131046 (2015).
Taieb, J., Zaanan, A., Le Malicot, K., Julié, C., Blons, H., Mineur, L. et al. Prognostic effect of BRAF and KRAS mutations in patients with stage III colon cancer treated with leucovorin, fluorouracil, and oxaliplatin with or without cetuximab: a post hoc analysis of the PETACC-8 trial. JAMA Oncol. 2, 643 (2016).
Jones, J. C., Renfro, L. A., Al-Shamsi, H. O., Schrock, A. B., Rankin, A., Zhang, B. Y. et al. Non-V600 BRAF mutations define a clinically distinct molecular subtype of metastatic colorectal cancer. J. Clin. Oncol. 35, 2624–2630 (2017).
Johnson, B., Loree, J. M., Morris, V. K., Dasari, A., Pant, S., Raghav, K. P. S. et al. Activity of EGFR inhibition in atypical (non-V600E) BRAF-mutated metastatic colorectal cancer. J. Clin. Oncol. 37(4 Suppl), 596–596 (2019).
Guinney, J., Dienstmann, R., Wang, X., de Reyniès, A., Schlicker, A., Soneson, C. et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. http://www.nature.com/nm/journal/vaop/ncurrent/full/nm.3967.html (2015). Cited 18 July 2018.
Taieb, J., Le Malicot, K., Shi, Q., Penault Lorca, F., Bouché, O., Tabernero, J. et al. Prognostic value of BRAF and KRAS mutations in MSI and MSS stage III colon cancer. J. Natl Cancer Inst. 109, djw272 (2017).
Richman, S. D., Seymour, M. T., Chambers, P., Elliott, F., Daly, C. L., Meade, A. M. et al. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J. Clin. Oncol. 27, 5931–5937 (2009).
Van Cutsem, E., Köhne, C.-H., Láng, I., Folprecht, G., Nowacki, M. P., Cascinu, S. et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J. Clin. Oncol. 29, 2011–2019 (2011).
Di Nicolantonio, F., Martini, M., Molinari, F., Sartore-Bianchi, A., Arena, S., Saletti, P. et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J. Clin. Oncol. 26, 5705–5712 (2008).
Safaee Ardekani, G., Jafarnejad, S. M., Tan, L., Saeedi, A. & Li, G. The prognostic value of BRAF mutation in colorectal cancer and melanoma: a systematic review and meta-analysis. PLoS ONE 7, e47054 (2012).
Heinemann, V., von Weikersthal, L. F., Decker, T., Kiani, A., Vehling-Kaiser, U., Al-Batran, S.-E. et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 15, 1065–1075 (2014).
Schwartzberg, L. S, Rivera, F, Karthaus, M, Fasola, G, Canon, J.-L. & Hecht, J. R. et al. PEAK: a randomized, multicenter phase ii study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J. Clin. Oncol. 32, 2240–2247 (2014).
Loupakis, F., Cremolini, C., Masi, G., Lonardi, S., Zagonel, V., Salvatore, L. et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N. Engl. J. Med. 371, 1609–1618 (2014).
Seligmann, J. F., Fisher, D., Elliott, F., Richman, S., Butler, R., Cheadle, J. et al. Exploring the poor outcomes of BRAF mutant (BRAF mut) advanced colorectal cancer (aCRC): analysis from 2,530 patients (pts) in randomized clinical trials (RCTs). J. Clin. Oncol. 33(15 Suppl), 3509–3509 (2015).
Wan, P. T., Garnett, M. J., Roe, S. M., Lee, S., Niculescu-Duvaz, D., Good, V. M. et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116, 855–867 (2004).
Barras, D., Missiaglia, E., Wirapati, P., Sieber, O. M., Jorissen, R. N., Love, C. et al. BRAF V600E mutant colorectal cancer subtypes based on gene expression. Clin. Cancer Res. 23, 104–115 (2017).
Corcoran, R. B., Ebi, H., Turke, A. B., Coffee, E. M., Nishino, M., Cogdill, A. P. et al. EGFR-mediated reactivation of MAPK signaling contributes to insensitivity of BRAF-mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2, 227–235 (2012).
Prahallad, A., Sun, C., Huang, S., Di Nicolantonio, F., Salazar, R., Zecchin, D. et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 483, 100–103 (2012).
Morris, V., Overman, M. J., Jiang, Z.-Q., Garrett, C., Agarwal, S., Eng, C. et al. Progression-free survival remains poor over sequential lines of systemic therapy in patients with BRAF-mutated colorectal cancer. Clin. Colorectal Cancer 13, 164–171 (2014).
Masi, G., Loupakis, F., Salvatore, L., Fornaro, L., Cremolini, C., Cupini, S. et al. Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: a phase 2 trial. Lancet Oncol. 11, 845–852 (2010).
Loupakis, F., Cremolini, C., Salvatore, L., Masi, G., Sensi, E., Schirripa, M. et al. FOLFOXIRI plus bevacizumab as first-line treatment in BRAF mutant metastatic colorectal cancer. Eur. J. Cancer 50, 57–63 (2014).
Cremolini, C., Loupakis, F., Antoniotti, C., Lupi, C., Sensi, E., Lonardi, S. et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 16, 1306–1315 (2015).
Bokemeyer, C., Van Cutsem, E., Rougier, P., Ciardiello, F., Heeger, S., Schlichting, M. et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur. J. Cancer 48, 1466–1475 (2012).
Phelip, J., Benhaim, L., Bouché, O., Christou, N., Desolneux, G., Dupré, A., et al. “Cancer colorectal métastatique”. Thésaurus National de Cancérologie Digestive. Thésaurus Natl Cancérologie Dig.; www.tncd.org (2019).
Benson, A. B., Venook, A. P., Al-Hawary, M. M., Cederquist, L., Chen, Y.-J., Ciombor, K. K. et al. NCCN guidelines insights: colon cancer, version 2.2018. J. Natl Compr. Cancer Netw JNCCN. 16, 359–369 (2018).
Ince, W. L., Jubb, A. M., Holden, S. N., Holmgren, E. B., Tobin, P., Sridhar, M. et al. Association of k-ras, b-raf, and p53 status with the treatment effect of bevacizumab. JNCI J. Natl Cancer Inst. 97, 981–989 (2005).
Price, T. J., Hardingham, J. E., Lee, C. K., Weickhardt, A., Townsend, A. R., Wrin, J. W. et al. Impact of KRAS and BRAF gene mutation status on outcomes from the phase III AGITG MAX trial of capecitabine alone or in combination with bevacizumab and mitomycin in advanced colorectal cancer. J. Clin. Oncol. 29, 2675–2682 (2011).
Wirapati, P., Pomella, V., Vandenbosch, B., Kerr, P., Maiello, E., Jeffery, G. M. et al. Velour trial biomarkers update: Impact of RAS, BRAF, and sidedness on aflibercept activity. J. Clin. Oncol. 35(15 Suppl), 3538–3538 (2017).
Yoshino, T., Obermannova, R., Bodoky, G., Prausová, J., Garcia-Carbonero, R., Ciuleanu, T.-E. et al. Are BRAF mutated metastatic colorectal cancer (mCRC) tumors more responsive to VEGFR-2 blockage? Analysis of patient outcomes by RAS/RAF mutation status in the RAISE study—a global, randomized, double-blind, phase III study. J. Clin. Oncol. 36(4 Suppl), 622–622 (2018).
Karapetis, C. S., Jonker, D., Daneshmand, M., Hanson, J. E., O’Callaghan, C. J., Marginean, C. et al. PIK3CA, BRAF, and PTEN status and benefit from cetuximab in the treatment of advanced colorectal cancer—results from NCIC CTG/AGITG CO.17. Clin. Cancer Res. 20, 744–753 (2014).
Peeters, M., Oliner, K. S., Price, T. J., Cervantes, A., Sobrero, A. F., Ducreux, M. et al. Analysis of KRAS/NRAS mutations in a phase III study of panitumumab with FOLFIRI compared with FOLFIRI alone as second-line treatment for metastatic colorectal cancer. Clin. Cancer Res. 21, 5469–5479 (2015).
Seymour, M. T., Brown, S. R., Middleton, G., Maughan, T., Richman, S., Gwyther, S. et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial. Lancet Oncol. 14, 749–759 (2013).
Pietrantonio, F., Petrelli, F., Coinu, A., Di Bartolomeo, M., Borgonovo, K., Maggi, C. et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur. J. Cancer 51, 587–594 (2015).
Rowland, A., Dias, M. M., Wiese, M. D., Kichenadasse, G., McKinnon, R. A., Karapetis, C. S. et al. Meta-analysis of BRAF mutation as a predictive biomarker of benefit from anti-EGFR monoclonal antibody therapy for RAS wild-type metastatic colorectal cancer. Br. J. Cancer 112, 1888–1894 (2015).
Geissler, M., Martens, U., Knorrenschield, R., Greeve, J., Florschuetz, A., Tannapfel, A. et al. 475O-mFOLFOXIRI + Panitumumab versus FOLFOXIRI as first-line treatment in patients with RAS wild-type metastatic colorectal cancer m(CRC): a randomized. Ann. Oncol. 28(Suppl 5), v158–v208 (2017).
Stintzing, S., Miller-Phillips, L., Modest, D. P., Fischer von Weikersthal, L., Decker, T., Kiani, A. et al. Impact of BRAF and RAS mutations on first-line efficacy of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab: analysis of the FIRE-3 (AIO KRK-0306) study. Eur. J. Cancer 79, 50–60 (2017).
Chapman, P. B., Hauschild, A., Robert, C., Haanen, J. B., Ascierto, P., Larkin, J. et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 (2011).
Gomez-Roca, C. A., Delord, J., Robert, C., Hidalgo, M., von Moos, R., Arance, A. et al. 535P Encorafenib (LGX818), an oral BRAF inhibitor, in patients with BRAF V600E metastatic colorectal cancer: results of dose expansion in an open-label, phase I study. Ann. Oncol. 25(Suppl 4), iv182–iv183 (2014).
Kopetz, S., Desai, J., Chan, E., Hecht, J. R., O’Dwyer, P. J., Lee, R. J. et al. PLX4032 in metastatic colorectal cancer patients with mutant BRAF tumors. J. Clin. Oncol. 28(15 Suppl), 3534–3534 (2010).
Kopetz, S., Desai, J., Chan, E., Hecht, J. R., O’Dwyer, P. J., Maru, D. et al. Phase II Pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J. Clin. Oncol. 33, 4032–4038 (2015).
Hyman, D. M., Puzanov, I., Subbiah, V., Faris, J. E., Chau, I., Blay, J.-Y. et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N. Engl. J. Med. 373, 726–736 (2015).
Corcoran, R. B., André, T., Atreya, C. E., Schellens, J. H. M., Yoshino, T., Bendell, J. C. et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAF V600E-mutant colorectal cancer. Cancer Discov. 8, 428–443 (2018).
Yaeger, R., Cercek, A., O’Reilly, E. M., Reidy, D. L., Kemeny, N., Wolinsky, T. et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clin. Cancer Res. 21, 1313–1320 (2015).
Hong, D. S., Morris, V. K., El Osta, B., Sorokin, A. V., Janku, F., Fu, S. et al. Phase IB study of vemurafenib in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with BRAFV600E mutation. Cancer Discov. 6, 1352–1365 (2016).
Kopetz, S., McDonough, S. L., Morris, V. K., Lenz, H.-J., Magliocco, A. M., Atreya, C. E. et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG 1406). J. Clin. Oncol. 35(4 Suppl), 520–520 (2017).
Mao, M., Tian, F., Mariadason, J. M., Tsao, C. C., Lemos, R., Dayyani, F. et al. Resistance to BRAF inhibition in BRAF-mutant colon cancer can be overcome with PI3K inhibition or demethylating agents. Clin. Cancer Res. 19, 657–667 (2013).
Elez, E., Schellens, J., Van Geel, R., Bendell, J., Spreafico, A., Schuler, M. et al. LBA-08 Results of a phase 1b study of the selective BRAF V600 inhibitor encorafenib in combination with cetuximab alone or cetuximab + alpelisib for treatment of patients with advanced BRAF-mutant metastatic colorectal cancer. Ann. Oncol. 26(Suppl 4), iv120 (2015).
Long, G. V., Stroyakovskiy, D., Gogas, H., Levchenko, E., de Braud, F., Larkin, J. et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N. Engl. J. Med. 371, 1877–1888 (2014).
Van Cutsem, E., Atreya, C., André, T., Bendell, J., Schellens, J., Gordon, M. et al. LBA-07 updated results of the MEK inhibitor trametinib (T), BRAF inhibitor dabrafenib (D), and anti-EGFR antibody panitumumab (P) in patients (pts) with BRAF V600E mutated (BRAFm) metastatic colorectal cancer (mCRC). Ann. Oncol. 26(Suppl 4), iv119 (2015).
Rawson, J. B., Mrkonjic, M., Daftary, D., Dicks, E., Buchanan, D. D., Younghusband, H. B. et al. Promoter methylation of Wnt5a is associated with microsatellite instability and BRAF V600E mutation in two large populations of colorectal cancer patients. Br. J. Cancer 104, 1906–1912 (2011).
Schadendorf, D., Hodi, F. S., Robert, C., Weber, J. S., Margolin, K., Hamid, O. et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J. Clin. Oncol. 33, 1889–1894 (2015).
Reck, M., Rodríguez-Abreu, D., Robinson, A. G., Hui, R., Csőszi, T., Fülöp, A. et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 375, 1823–1833 (2016).
Lapeyre-Prost, A., Terme, M., Pernot, S., Marcheteau, E., Pointet, A.-L., Voron, T. et al. Immune therapy in colorectal cancer. Colorec. Cancer 6, 1–10 (2017).
Weisenberger, D. J., Siegmund, K. D., Campan, M., Young, J., Long, T. I., Faasse, M. A. et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat. Genet. 38, 787–793 (2006).
English, D. R., Young, J. P., Simpson, J. A., Jenkins, M. A., Southey, M. C., Walsh, M. D. et al. Ethnicity and risk for colorectal cancers showing somatic BRAF V600E mutation or CpG island methylator phenotype. Cancer Epidemiol Biomarkers Prev. 17, 1774–1780 (2008).
Venderbosch, S., Nagtegaal, I. D., Maughan, T. S., Smith, C. G., Cheadle, J. P., Fisher, D. et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin. Cancer Res. 20, 5322–5330 (2014).
Lochhead, P., Kuchiba, A., Imamura, Y., Liao, X., Yamauchi, M., Nishihara, R. et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. JNCI J. Natl Cancer Inst. 105, 1151–1156 (2013).
Rosenbaum, M. W., Bledsoe, J. R., Morales-Oyarvide, V., Huynh, T. G. & Mino-Kenudson, M. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod. Pathol. 29, 1104–1112 (2016).
Overman, M. J., McDermott, R., Leach, J. L., Lonardi, S., Lenz, H.-J., Morse, M. A. et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 18, 1182–1191 (2017).
Overman, M. J., Lonardi, S., Wong, K. Y. M., Lenz, H.-J., Gelsomino, F., Aglietta, M. et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability–high metastatic colorectal cancer. J. Clin. Oncol. 36, 773–779 (2018).
Frederick, D. T., Piris, A., Cogdill, A. P., Cooper, Z. A., Lezcano, C., Ferrone, C. R. et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin. Cancer Res. 19, 1225–1231 (2013).
Van Cutsem, E., Cuyle, P.-J., Huijberts, S., Yaeger, R., Schellens, J. H. M., Elez, E. et al. BEACON CRC study safety lead-in (SLI) in patients with BRAFV600E metastatic colorectal cancer (mCRC): efficacy and tumor markers. J. Clin. Oncol. 36(4 Suppl), 627–627 (2018).
Samowitz, W. S., Sweeney, C., Herrick, J., Albertsen, H., Levin, T. R., Murtaugh, M. A. et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 65, 6063–6069 (2005).
Roth, A. D., Tejpar, S., Delorenzi, M., Yan, P., Fiocca, R., Klingbiel, D. et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J. Clin. Oncol. 28, 466–474 (2010).
Gavin, P. G., Colangelo, L. H., Fumagalli, D., Tanaka, N., Remillard, M. Y., Yothers, G. et al. Mutation profiling and microsatellite instability in stage II and III colon cancer: an assessment of their prognostic and oxaliplatin predictive value. Clin. Cancer Res. 18, 6531–6541 (2012).
André, T., de Gramont, A., Vernerey, D., Chibaudel, B., Bonnetain, F., Tijeras-Raballand, A. et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J. Clin. Oncol. 33, 4176–4187 (2015).
Sinicrope, F. A., Mahoney, M. R., Smyrk, T. C., Thibodeau, S. N., Warren, R. S., Bertagnolli, M. M. et al. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J. Clin. Oncol. 31, 3664–3672 (2013).
Modest, D. P., Ricard, I., Heinemann, V., Hegewisch-Becker, S., Schmiegel, W., Porschen, R. et al. Outcome according to KRAS-, NRAS-and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann. Oncol. 27, 1746–1753 (2016).
Innocenti, F., Ou, F.-S., Qu, X., Zemla, T. J., Niedzwiecki, D., Tam, R. et al. Mutational analysis of patients with colorectal cancer in CALGB/SWOG 80405 identifies new roles of microsatellite instability and tumor mutational burden for patient outcome. J. Clin. Oncol. 37, 1217–1227 (2019).
Falchook, G. S., Long, G. V., Kurzrock, R., Kim, K. B., Arkenau, T. H., Brown, M. P. et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet 379, 1893–1901 (2012).
van Geel, R. M. J. M., Tabernero, J., Elez, E., Bendell, J. C., Spreafico, A., Schuler, M. et al. A Phase Ib dose-escalation study of encorafenib and cetuximab with or without alpelisib in metastatic BRAF -mutant colorectal cancer. Cancer Discov. 7, 610–619 (2017).
Tabernero, J., Geel, R. V., Guren, T. K., Yaeger, R. D., Spreafico, A., Faris, J. E. et al. Phase 2 results: encorafenib (ENCO) and cetuximab (CETUX) with or without alpelisib (ALP) in patients with advanced BRAF-mutant colorectal cancer (BRAFm CRC). J. Clin. Oncol. 34(15 Suppl), 3544–3544 (2016).
Author information
Authors and Affiliations
Contributions
J.T. and A.L.P. have designed the review, performed the bibliographical research and wrote the paper, they have contributed equally to this work. A.Z. and P.L.P. have participated in paper writing, critical corrections and improvement and to figures design.
Corresponding author
Ethics declarations
Competing interests
J.T. declared providing an advisory role for Roche, Merck, KGaA, Amgen Lilly, Baxalta, Servier and Sirtex Medical. A.L.P. declared a consultancy role for MERCK, MERCK SERONO and AMGEN/COHESIA. A.Z. had a consultancy role for Amgen, Baxter, Lilly, Merck Serono, MSD, Roche, Sanofi and Servier. P.L.P. declared a consultancy role for Amgen, Astrazeneca, Biocartis, Boehringer-Ingelheim, Merck, MSD, BMS, Roche and Sanofi.
Ethics approval and consent to participate
Not applicable
Funding
No sources of funding are to be reported for this study.
Consent to publish
Not applicable
Data availability
Not applicable
Note
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY 4.0).
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Taieb, J., Lapeyre-Prost, A., Laurent Puig, P. et al. Exploring the best treatment options for BRAF-mutant metastatic colon cancer. Br J Cancer 121, 434–442 (2019). https://doi.org/10.1038/s41416-019-0526-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-019-0526-2
This article is cited by
-
The role of heavy metals in the development of colorectal cancer
BMC Cancer (2023)
-
Liver Transplantation for Nonresectable Colorectal Liver Metastases (CRLM)
Indian Journal of Surgical Oncology (2023)
-
Nursing care and management of adverse events for patients with BRAFV600E-mutant metastatic colorectal cancer receiving encorafenib in combination with cetuximab: a review
Supportive Care in Cancer (2023)
-
BRAFV600E Metastatic Colorectal Cancer: Perspective from a Patient, a Caregiver, and an Oncologist
Advances in Therapy (2023)
-
BRAF-V600E-Testung beim metastasierten kolorektalen Karzinom und neue, chemotherapiefreie Therapieoptionen
Der Pathologe (2021)