Abstract
Background
To report retinal pigment epithelium (RPE) apertures as a possible evolution of serous pigment epithelium detachment (PED) in patients with chronic central serous chorioretinopathy (CSC) and to analyze their progression over time.
Design
Retrospective case series.
Methods
Fifteen patients (17 eyes) with a diagnosis of avascular PED in chronic CSC were retrospectively evaluated based on multimodal imaging. All patients had documented records of clinical examination, best correct visual acuity (BCVA), fundus autofluorescence (FAF), fluorescein and indocyanine green angiography, spectral-domain optical coherence tomography, and optical coherence tomography angiography (OCT-A). Primary outcomes measures were RPE aperture imaging analysis along with their temporal course.
Results
All eyes showed the RPE aperture as an interruption of the RPE in correspondence of the PED with neither sign of rippling nor retraction. Mean age was 59.4 ± 8.1 years and mean BCVA was 0.34 ± 0.24 logMAR. In all eyes, FAF displayed a well-circumscribed roundish hypoautofluorescence. Multimodal imaging and OCT-A confirmed the absence of any vascular network beneath the aperture in all study eyes. The mean time of follow-up was 19.3 ± 14.3 months, and RPE discontinuities showed an increase in size over time from 0.81 ± 0.39 mm2 to 0.95 ± 0.45 (P = 0.005).
Conclusions
RPE aperture is a new finding in the setting of chronic CSC and it should be distinguished by RPE tears for the different pathogenesis and evolution in time.
Similar content being viewed by others
Introduction
Central serous chorioretinopathy (CSC) is a retinal disorder typically affecting young and middle-aged adults, with a higher incidence in men [1, 2]. It is characterized by serous detachment of the neurosensory retina, often accompanied by retinal pigment epithelium detachments (PEDs), that tend to resolve between 4 and 6 months; albeit in 31% of cases recurrences may occur [3,4,5]. In 5% of patients, subretinal fluid persists along with pigmentary changes and patchy retinal pigment epithelium (RPE) atrophy resulting in a longstanding disease [6, 7].
Guyer et al. demonstrated by means of indocyanine green videoangiography that PED is commonly (75%) noted in CSC [8]. Recently Ersoz et al. analyzed patient characteristics and risk factors in a large cohort of 811 patients with CSC, reporting a prevalence of RPE detachment of 83% with a higher frequency in the chronic forms [9].
Dissimilar to age-related macular degeneration (AMD), RPE tears represent a rare complication in CSC due to the different nature of the PED which are mostly avascular [10,11,12]. In 2016, Querques et al. described RPE aperture as a previously unreported finding in the evolution of avascular PED in AMD, defined as discontinuity of the RPE with no signs of rippling and retraction [13].
Later some authors confirmed the presence of atypical RPE defects in AMD not attributable to geographic atrophy or RPE tears [14], and more recently Bansal et al. reported this new finding in foveomacular vitelliform dystrophy [15]. The aim of the study was to describe the imaging characteristics of RPE apertures in the setting of chronic CSC and to analyze their progression over time.
Methods
Patients and study population
Medical records of patients with chronic CSC were retrospectively evaluated. Subjects were recruited from the Retina service of the Eye Clinic, University of Cagliari, Italy, and the L.V. Prasad Eye Institute, Hyderabad, Telangana, India.
The study was approved by the two local institutional review committee and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all subjects. Inclusion criteria were: age >18 years old, patients classified as having history of chronic CSC (for at least 6 months in duration) showing discontinuities of the RPE in correspondence of serous PED confirmed by multimodal imaging evaluation, absence of neovascularization beneath the PED ascertained by fluorescein and indocyanine green angiography (FA and ICGA), spectral-domain optical coherence tomography (SD-OCT) and optical coherence tomography angiography (OCT-A), and minimum follow-up of 6 months. Patients not showing subretinal fluid at the moment of the RPE aperture formation were included as well.
Exclusion criteria were: presence of other concomitant ocular diseases in the study eye (age-related macular degeneration, diabetic retinopathy, high myopia, vein, or artery occlusion), vitreoretinal surgery and/or treatments including intravitreal injections of anti-vascular endothelial grow factor (IVT), argon laser, or photodynamic therapy (PDT) prior to the aperture onset.
Imaging
All patients underwent a complete ophthalmologic examination encompassing best-corrected visual acuity (BCVA) measurement in Snellen with logMAR conversion for statistical analysis, fundus autofluorescence (FAF), FA, ICGA, and SD-OCT (Heidelberg Spectralis HRA + OCT, Heidelberg Engineering). OCT-A was performed using swept source DRI OCT-plus (Triton, Topcon, Tokyo, Japan) and Angiovue (Optovue, Fremont, California, USA). All imaging data were retrospectively reviewed by two retinal specialists (E.P. and J.C.) who analyzed qualitatively all FAF, SD-OCT, FA, and ICGA images.
The temporal course of RPE apertures was evaluated by manually tracing the corresponding area shown in FAF using the built-in software (Spectralis Acquisition and Viewing Modules, version 5.6.1.0; Heidelberg Engineering) at different visits.
Maximum height of the PED, presence or absence of subretinal fluid and subfoveal choroidal thickness were recorded at baseline and at the last follow-up. The eyes were either managed by observation or received treatment in case of clinically significant subretinal and intraretinal fluid including oral medications (eplerenone) or PDT. Patients treated with PDT with verteporfin (Novartis AG, Basel, Switzerland) followed the protocol of the Treatment of AMD with Photodynamic Therapy (TAP) Study [16], receiving a full fluence treatment.
Statistical analysis
Statistical analysis was conducted with SPSS statistical software (SPSS Inc, Chicago, Illinois, USA). Values are expressed as mean ± standard deviation (SD). Results of descriptive analyses are expressed as counts and percentages for categorical variables, and as means ± standard deviations for quantitative variables. The Wilcoxon test was used to compare continuous variables at the first and last follow-up visit. The McNemar test was used to compare dichotomous variables at the first and last follow-up visit. The Mann–Whitney U test was used to compare continuous variables in eyes with or without subretinal fluid, with macular or extra-macular RPE apertures and RPE apertures located at apex or at the base of the PED.
Results
Overall, complete medical records of 160 patients with CSC were retrospectively evaluated. In total, 17 eyes of 15 patients (11 males and 4 females) met the inclusion criteria and were included in the study. All patients involved showed clinical signs for at least 6 months indicating a chronic CSC. Two patients had bilateral RPE aperture (Fig. 1). Mean age ± SD at presentation was 59.4 ± 8.1 years. Baseline BCVA ranged from 0.1 to 1 logMAR with a mean value of 0.34 ± 0.24 logMAR and did not change significantly at the end of follow-up (P = 0.222, Table 1).
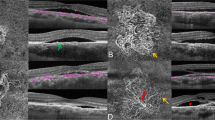
Multimodal imaging of a 62-year-old woman with chronic CSC and bilateral RPE aperture. a, b FAF showing two well-demarcated hypoautofluorescent areas (arrowhead), a large one in macular area and smaller one along the superotemporal vascular arcade in the right and left eye, respectively, corresponding to RPE apertures. c–f FA and ICGA revealing clinical signs of chronic CSC with a mottled hyperfluorescence (FA) and hypofluorescence (ICGA) due to the RPE decompensation with two well-circumscribed window defects areas in correspondence of the RPE aperture in both eyes. g, h OCT angiography images of choriocapillaris segmentation with corresponding B-scan confirming the absence of any vascular network below the PED bilaterally. i, j SD-OCT horizontal line scans passing through the PEDs (shown in FA) displaying the RPE apertures as discontinuities of the RPE (asterisks) with increased back scattering in both eyes, with intraretinal cysts changes in the right eye (i)
In 13 study eyes (76.5%) apertures were visible at presentation on multimodal imaging, whereas in 4 eyes the RPE discontinuity was developed after a variable interval of time from the diagnosis of PED, with a mean time of 13.5 ± 3.1 months (Fig. 2). The mean time of follow-up was 19.3 ± 14.3 months (range from 6 to 42 months). The included eyes showed no evidence of choroidal neovascularization either at presentation or during the follow-up, as confirmed by multimodal imaging and OCT-A.
Multimodal imaging of the right eye of a 58-years old man with chronic CSC before and after the development of RPE aperture. a Late-phase FA and b ICGA showing some areas of hyperfluorescence at the posterior pole with a window defect area inferior to the macula. c FAF revealing typical features of CSC with mottled areas of hyperautofluorescence along the superotemporal vascular arcade. d SD-OCT horizontal line scan disclosing a serous PED with a thinning of the RPE at its right bottom margin. e, f FAF and SD-OCT 13 moths later showing the onset of the RPE aperture as a small hypoautofluorescent area at the right border of the PED (FAF) with the characteristic RPE discontinuity (white asterisks) and appearance of subretinal fluid (SD-OCT)
RPE apertures were localized mostly in macular area (12 eyes, 70.6%) and appeared on FAF as well-circumscribed areas of hypoautofluorescence and on FA and ICGA as demarcated round areas of window defects. SD-OCT disclosed discontinuity of the RPE mostly at the apex (13 eyes, 76.5%) or at the base (4 eyes, 23.5%) of the PED, with no sign of torn or rippling. OCT-A depicted no abnormal flow beneath the PED in b-scan and on en face visualization showed a roundish area of hyperreflectivity (choroidal vessels) in correspondence of the aperture owing to the RPE absence (Figs. 1 and 3). The PED height reduced significantly during the follow-up period from 207.5 ± 109.1 µm to 166.8 ± 87.4 (P = 0.001). On the contrary RPE aperture areas measured on FAF enlarged homogeneously from 0.81 ± 0.39 mm2 to 0.95 ± 0.45 (P = 0.005, Table 1). Patients with subretinal fluid at the baseline showed a significantly lower BCVA, a significantly higher PED maximal height, and a significantly larger area of RPE aperture than patients with no subretinal fluid (0.48 ± 0.24 logMAR vs 0.19 ± 0.10, P = 0.006; 275.2 ± 108.0 µm vs 131.3 ± 36.2, P = 0.004; 0.99 ± 0.34mm2 vs 0.60 ± 0.35, P = 0.033; respectively). Moreover, patients with no subretinal fluid at the last follow-up visit showed a significantly higher decrease in the height of PED if compared to those with subretinal fluid (53.9 ± 48.8 µm vs 9.0 ± 14.4; P = 0.027). Conversely, BCVA, maximal height of the PED and area of RPE aperture showed no significant differences in eyes with RPE apertures located either at apex or at the base of the PED (P > 0.05). Mean subfoveal choroidal thickness was 373.4 ± 51.3 at baseline and 375.4 ± 49.9 at the last follow-up, with no statistical differences (P = 0.943). No significant differences in CT were found between patients with and without subretinal fluid at baseline (370.4 ± 69.1 vs 376.1 ± 32.6, P = 0.564), and at the last follow-up (391.8 ± 71.3 vs 368.5 ± 39.9, P = 0.673). Furthermore, no significant associations were found between choroidal thickness and PED height, area and location of RPE apertures (all P > 0.05).
Multimodal imaging of the left eye of a 54-year-old man with treatment naïve chronic CSC. a FAF showing the RPE aperture (arrowhead) as a well-circumscribed hypoautofluorescent macular area with a wide hyperautofluorescence nasally and a crescent-shaped faint hypoautofluorescence below. b, c Late-phase FA and ICGA revealing hyperfluorescent areas with some leaking points, and a perimacular window defect area. d SD-OCT horizontal line scan confirming intraretinal and subretinal fluid, with RPE aperture in correspondence of the PED (white asterisks). Six months later, after a full-fluence PDT session. e FAF showing the enlargement of the hypofluorescent macular area (arrowhead) and the transition from a faint hypoautofluorescence to a slight hyperautofluorescence inferior to the macula due to the subretinal fluid reabsorption. f SD-OCT revealing a complete resolution of subretinal fluid, with small intraretinal cysts and a flattening of the PED associated to a complete disappearance of the RPE (arrows). g–i OCT angiography image with corresponding B-scan displaying a roundish area of choroidal vessels beneath the RPE aperture as a result of a window defect
Two patients underwent PDT due to a clinically significant amount of subretinal and intraretinal fluid with choroidal leakage on ICGA showing a resolution of the fluid after 6 months (Fig. 3).
Discussion
CSC is an interesting retinal disorder challenging many retinal specialists for the variability of clinical manifestations. Although PED has a well-known association with CSC, the presence of RPE aperture as a possible late complication of the RPE elevations has never been reported. Choroidal hyperpermeability, increased choriocapillaris leakage and the alteration of the RPE pump, play a key role in the fluid accumulation between the basement membrane of the RPE and the inner collagenous layer of Bruch membrane in CSC [17].
Typically, in these kind of patients serous PEDs are mostly avascular and rarely complicated by CNV [9, 18, 19], and unlike vascularized PED, the incidence and progression of RPE tears is considerably lower owing to the absence of the contraction forces from the neovascular tissue [20,21,22].
In our series, the RPE apertures did not show any sign of tear or rippling on SD-OCT and did not involve the overlying photoreceptor-layer. As shown in Fig. 2, there was a first focal thinning of the RPE followed by an abrupt discontinuity of the latter increasing over the time, suggesting a suffering of the epithelial cells anticipating the RPE aperture onset. In addition, RPE tears tend to be stable and not to enlarge after their onset, once the tractional forces cease to act [23]. Conversely, when aperture first appears, the hydrostatic pressure from below the PED continues to exist leading to an increasing in size. However, in all study eyes PED tended to flatten (PED maximum height decreased from 207.5 ± 109.1 µm to 166.8 ± 87.4, P = 0.001) following the development of the aperture as to indicate a slight reduction of the hydrostatic forces.
On the contrary, it could be possible that the flattening of PED could be itself the result of the hydrostatic pressure. Worthy of note, eyes with subretinal fluid at the first visit showed a significantly worse BCVA, a significantly higher PED maximal height, and a significantly larger area of RPE aperture (P < 0.05). It can be speculated that the longstanding fluid below and above the PED places the RPE cells to a higher risk of damage. Alternatively, it is possible that the subretinal fluid secondly increase because of the local loss of RPE cells resulting in an inability to pump ions and fluid out of the subretinal space. In our series, the BCVA reduced from a mean of 0.34 ± 0.24 to 0.36 ± 0.25 logMar (P = 0.222) during the follow-up, however there being no statistically significant difference. The relatively good visual prognosis along with the sparing of the photoreceptors-layer in most of cases, seem to confirm the hypothesis of some authors for which photoreceptors would be able to survive even without normal RPE [24, 25].
Analysis of FAF images revealed signal changes in correspondence of the aperture consisting in hyperfluorescent halo surrounding the site of aperture in most of cases, confirming the transition from a functional RPE to a missing one. Interestingly, RPE aperture occurs only in eyes with chronic CSC, as a result of a longstanding suffering of the pigment epithelial cells. A more advanced mean age of the study patients seems to support this hypothesis. The development process of RPE aperture is not yet completely understood, notwithstanding we found many similarities with all findings reported by Querques et al. in AMD [13], as for the absence of any retraction or rippling of the RPE and the progressive enlargement of aperture areas.
Albeit a complete multimodal imaging evaluation is necessitated to identify all clinical features of CSC and OCT-A is very helpful in confirming the absence of CNV beneath the PED, it is our belief that RPE aperture diagnosis strongly relies on SD-OCT.
Weaknesses of this study include its retrospective nature and the small number of patients included. In addition, it was not possible to evaluate the influence of the PDT procedure on the natural course of the RPE aperture considering that only two eyes received the treatment. The actual nature of apertures remains unclear, perhaps they represent a deterioration of the RPE but not necessary a true loss of tissue. The lack of autofluorescence on FAF and the window defect on FA/ICGA indicate a loss of pigment, but not necessarily an absence of the layer. Further clinicopathologic correlations are needed. In conclusion, RPE aperture may be seen in chronic CSC as a possible evolution of longstanding avascular PEDs. Its recognition is important considering the differences in terms of development and natural course with RPE tears over time.
Summary
What was known before
-
Pigment epithelium detachments represent a common finding in chronic CSC.
-
Retinal pigment epithelium shows different kind of alterations in CSC.
-
Retinal pigment epithelium tears are a rare feature in the setting of CSC.
What this study adds
-
Retinal pigment epithelium aperture can occur in patients with chronic CSC in correspondence of avascular pigment epithelium detachments (PEDs).
-
Unlike Retinal pigment epithelium tears, aperture tend to increase in size over time.
References
Liew G, Quin G, Gillies M, Fraser-Bell S. Central serous chorioretinopathy: a review of epidemiology and pathophysiology. Clin Exp Ophthalmol. 2013;41:201–14.
Yannuzzi LA. Type-a behavior and central serous chorioretinopathy. Retina. 1987;7:111–31.
Spaide RF, Campeas L, Haas A, Yannuzzi LA, Fisher YL, Guyer DR, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996;103:2070–80.
Haimovici R, Koh S, Gagnon DR, Lehrfeld T, Wellik S. Risk factors for central serous chorioretinopathy: a case-control study. Ophthalmology. 2004;111:244–9.
Tittl MK, Spaide RF, Wong D, Pilotto E, Yannuzzi LA, Fisher YL, et al. Systemic findings associated with central serous chorioretinopathy. Am J Ophthalmol. 1999;128:63–8.
Schatz H, Madeira D, Johnson RN, McDonald HR. Central serous chorioretinopathy occurring in patients 60 years of age and older. Ophthalmology. 1992;99:63–7.
Castro-Correia J, Coutinho MF, Rosas V, Maia J. Long-term follow-up of central serous retinopathy in 150 patients. Doc Ophthalmol. 1992;81:379–86.
Guyer DR, Yannuzzi LA, Slakter JS, Sorenson JA, Ho A, Orlock D. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994;112:1057–62.
Ersoz MG, Arf S, Hocaoglu M, Sayman Muslubas I, Karacorlu M. Patient characteristics and risk factors for central serous chorioretinopathy: an analysis of 811 patients. Br J Ophthalmol. 2018; e-pub ahead of print 12 Jul 2018. https://doi.org/10.1136/bjophthalmol-2018-312431.
Goldstein BGPP. “Blow-outs” in the retinal pigment epithelium. Br J Ophthalmol. 1987;71:676–81.
Jo Y-J, Cho C-B, Lee S-B, Kim J-Y, Kim W-J, Iwase T. Bilateral central serous chorioretinopathy with retinal pigment epithelium tears following epidural steroid injection. Indian J Ophthalmol. 2013;61:514–5.
Ishida Y, Kato T, Minamoto A, Yokoyama T, Jian K, Mishima HK. Retinal pigment epithelial tear in a patient with central serous chorioretinopathy treated with corticosteroids. Retina. 2004;24:633–6.
Querques G, Capuano V, Costanzo E, Corvi F, Querques L, Introini U, et al. Retinal pigment epithelium aperture: a previously unreported finding in the evolution of avascular pigment epithelium detachment. Retina. 2016;36:S65–72.
Giannakaki-zimmermann H, Querques G, Munch IC, Shroff D, Sarraf D, Chen X, et al. Atypical retinal pigment epithelial defects with retained photoreceptor layers: a so far disregarded finding in age related macular degeneration. BMC Ophthalmol. 2017;17:67.
Bansal R, Yangzes S, Singh R, Katoch D, Dogra MR, Gupta V, et al. Retinal pigment epithelium aperture: a late-onset complication in adult-onset foveomacular vitelliform dystrophy. Indian J Ophthalmol. 2018;66:83–8.
Anon. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials–TAP report. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Arch Ophthalmol. 1999;117:1329–45.
Tan ACS, Simhaee D, Balaratnasingam C, Dansingani KK, Yannuzzi LA. A perspective on the nature and frequency of pigment epithelial detachments. Am J Ophthalmol. 2016;172:13–27.
Bandello F, Virgili G, Lanzetta P, Pirracchio A, Menchini U. [ICG angiography and retinal pigment epithelial decompensation (CRSC and epitheliopathy)]. J Fr Ophtalmol. 2001;24:448–51.
Peiretti E, Ferrara DC, Caminiti G, Mura M, Hughes J. Choroidal neovascularization in caucasian patients with longstanding central serous chorioretinopathy. Retina. 2015;35:1360–7.
Chang LK, Flaxel CJ, Lauer AK, Sarraf D. RPE tears after pegaptanib treatment in age-related macular degeneration. Retina. 2007;27:857–63.
Cunningham ET, Feiner L, Chung C, Tuomi L, Ehrlich JS. Incidence of retinal pigment epithelial tears after intravitreal ranibizumab injection for neovascular age-related macular degeneration. Ophthalmology. 2011;118:2447–52.
Gutfleisch M, Heimes B, Schumacher M, Dietzel M, Lommatzsch A, Bird A, et al. Long-term visual outcome of pigment epithelial tears in association with anti-VEGF therapy of pigment epithelial detachment in AMD. Eye. 2011;25:1181–6.
Mrejen S, Sarraf D, Srikrishna M, Bailey FK. Multimodal imaging of pigment epithelial detachment. Retina. 2013;33:1735–62.
Bressler NM, Finklestein D, Sunness JS, Maguire AM, Yarian D. Retinal pigment epithelial tears through the fovea with preservation of good visual acuity. Arch Ophthalmol. 1990;108:1694–7.
Curcio CA, Millican CL, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci. 1993;34:3278–96.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iovino, C., Chhablani, J., Parameswarappa, D.C. et al. Retinal pigment epithelium apertures as a late complication of longstanding serous pigment epithelium detachments in chronic central serous chorioretinopathy. Eye 33, 1871–1876 (2019). https://doi.org/10.1038/s41433-019-0505-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-019-0505-0
This article is cited by
-
Photodynamic therapy as a treatment option for peripapillary pachychoroid syndrome: a pilot study
Eye (2022)
-
Comment on: ‘Retinal pigment epithelium apertures as a late complication of longstanding serous pigment epithelium detachments in chronic central serous chorioretinopathy’
Eye (2022)
-
Imaging of a retinal pigment epithelium aperture using polarization-sensitive optical coherence tomography
Japanese Journal of Ophthalmology (2021)