Abstract
Background/Objectives
To evaluate seasonal fluctuations in intraocular pressure (IOP) in primary open-angle glaucoma (POAG) and its associated factors.
Subjects/Methods
POAG patients treated only with glaucoma eye drops were enroled. Winter and summer IOPs were evaluated. The Seasonal fluctuation rate of IOP was defined as follows: (mean winter IOP—mean summer IOP)/mean IOP in all seasons. Multiple linear regression analysis was used to explore factors associated with the seasonal IOP fluctuation rate including: age, gender, family history of glaucoma, type of glaucoma, number of eye drops, type of eye drops, mean deviation (MD) value, MD slope, disc haemorrhage, central corneal thickness and spherical equivalent.
Results
Winter IOP was higher than summer IOP in 204 POAG eyes of 204 patients, including 162 eyes with normal tension glaucoma (NTG) (13.2 ± 2.7 vs. 12.0 ± 2.3 mmHg, P < 0.001). The mean age and follow-up duration were 63.3 ± 11.4 years and 140.0 ± 66.9 months. Initial MD and MD slope were −2.1 ± 3.4 dB and −0.07 ± 0.50 dB/year, respectively. POAG was positively associated with the rate of seasonal IOP fluctuations compared to NTG (β = 5.29, P = 0.013). Family history, and timolol and carteolol use were also factors associated with the IOP fluctuation rate (β = −6.27, P = 0.007; β = 4.94, P = 0.030; and β = 4.51, P = 0.042, respectively).
Conclusions
We confirmed seasonal IOP fluctuations in POAG. Type of glaucoma, family history of glaucoma, and β-blocker use might influence IOP fluctuations.
Similar content being viewed by others
Introduction
Glaucoma is the second leading cause of blindness worldwide [1]. It is estimated that the total number of glaucoma cases worldwide will rise to 111.8 million by 2040 [2]. Intraocular pressure (IOP) is the most important and only clinically modifiable risk factor for progression of glaucoma [3]. At present, the only proven treatment for glaucoma is reduction of IOP.
IOP is not a fixed value; it changes over time [4]. We have previously demonstrated that night-time IOP increased in many cases of primary open-angle glaucoma (POAG) [5]. Various types of fluctuations have been reported, including 24-hour diurnal fluctuation [6] and long-term fluctuation [7]. Long-term IOP fluctuation is often defined as the standard deviation of IOP measurements across all visits. Although still controversial, these IOP fluctuations have been reported to contribute to the development and progression of glaucoma [4, 8]. Therefore, it is clinically important to figure out the details of IOP fluctuations in glaucoma patients.
Previous studies on seasonal fluctuations in IOP have been conducted. Qureshi et al. showed significant seasonal fluctuations in both normal subjects and patients with ocular hypertension (OHT) [9, 10]. In a large-scale study by Gardiner et al., the IOP fluctuations in OHT were examined in six climatically similar geographic regions. They showed that winter IOPs were higher than summer IOPs in all regions, although the magnitude of the fluctuations varied from region to region [11]. Ayaki et al. focused on POAG with dry eye syndrome, which is known to be worse in winter, and revealed significant seasonal fluctuations [12]. They suggested that the disturbance of the ocular surface and associated inflammation increased in winter and contributed to increased IOP. Significantly higher IOP was also observed by Mansouri et al. with a novel implantation device in POAG eyes during winter compared to summer [13]. Recently, we demonstrated that seasonal IOP fluctuations had an impact on retinal nerve fibre layer thinning in POAG eyes [14]. These findings indicated that seasonal fluctuations might be clinically important as with other types of IOP fluctuations.
Several factors associated with seasonal IOP fluctuations have been reported. Cheng et al. [15]. indicated that air temperature and sunlight exposure were independent factors. Ayaki et al. [12]. considered that dry-eye syndrome increased winter IOP. Gardiner et al. [11]. suggested that IOP-lowering eye drops led to a reduction in seasonal IOP fluctuations in OHT. However, whether other candidate factors such as age, gender, family history, type of glaucoma, and type of eye drops influence the fluctuation has not been fully explored.
In this study, we aimed to elucidate the details of seasonal IOP fluctuation in POAG eyes, including normal tension glaucoma (NTG). We evaluated winter and summer IOP in a large number of patients undergoing long-term glaucoma treatment and explored any factors affecting the seasonal fluctuation using their demographic and clinical data.
Materials and methods
This study was approved by the ethics committee of the Jikei University School of Medicine [approval number: 31-487(10069)]. The study design followed the tenets of the Declaration of Helsinki. Informed consent from patients was obtained through an opt-out method.
POAG patients who visited the Jikei University School of Medicine between July and December 2019 were retrospectively reviewed. All of the patients underwent a comprehensive ophthalmologic examination by a glaucoma specialist (TN), including best-corrected visual acuity with the Landolt C chart, measurements of IOP by Goldmann applanation tonometry (GAT), slit-lamp biomicroscopy, gonioscopy, measurements of central corneal thickness by corneal pachymetry, dilated ophthalmoscopy, fundus photography, measurements of visual fields by Humphrey Visual Field Analyzer (HFA, Carl Zeiss Meditec, Dublin, CA), and measurements of retinal nerve fibre layer (RNFL) thickness by Cirrus HD-optical coherence tomography (OCT) (Carl Zeiss Meditec, Dublin, CA).
The diagnosis of POAG was based on the criteria of a previous population-based study [16]. Glaucomatous optic neuropathy and visual field defects consistent with optic changes were evaluated. Glaucomatous optic neuropathy was diagnosed to be present when: (1) a vertical cup-to-disc ratio of the optic nerve head was 0.7 or greater, (2) a rim-to-disc ratio at the superior portion (11-1 o’clock positions) or inferior portion (5-7 o’clock positions) of 0.1 or less, (3) a difference in the vertical cup-to-disc ratio of 0.2 or more between both eyes, or (4) a RNFL defect was found. Visual field sensitivity was measured using HFA 30-2 SITA Standard program. Based on the Anderson-Patella’s criteria, glaucomatous visual field defect was diagnosed to be present when: (1) the glaucoma hemi-field test results were outside normal limits, (2) pattern deviation probability plots, in the upper or lower hemifield, showed a cluster of three or more non-edge contiguous points having sensitivity with a probability of less than 5%, of which at least one point has a probability of less than 1%, or (3) a pattern standard deviation outside of the 95% normal confidence limits.
We enroled POAG patients treated with only topical medication (glaucoma eye drops), undergoing GAT both in winter (December to February) and summer (July to September). Subjects with other ocular diseases affecting visual acuity or/and visual field, a history of laser or/and surgical treatment for glaucoma, decimal visual acuity worse than 0.7, or an HFA mean deviation (MD) value at first visit of worse than −15.0 dB were excluded. Based on reliability indices of HFA, we also excluded patients whose fixation losses exceeded 20%, or whose false-positive or false-negative errors exceeded 33%. Regarding RNFL thickness measurements, only patients having reliable OCT images with a signal strength of six or greater, no segmentation error, no motion artefact, and appropriate centration were included. When both patient eyes met the selection criteria, one eye was enroled at random.
Monthly mean air temperature in Tokyo, Japan from 2015 to 2018 was obtained from the Japanese Meteorological Agency website (https://www.jma.go.jp/jma/indexe.html) (Fig. 1). Based on these data, December to February was defined as winter and July to September as summer. IOP of POAG eyes was measured by GAT at every visit to calculate the means during winter and summer. The seasonal fluctuation rate of IOP was calculated as follows: (mean winter IOP—mean summer IOP)/mean IOP in all seasons.
Continuous variables were presented as means ± standard deviations (range). The decimal visual acuity obtained from the Landolt C chart was converted to the logarithm of the minimum angle of resolution (logMAR) scale. An independent t test or Mann-Whitney U test was used to compare continuous variables between two groups after data normality was assessed. Chi-square tests were used to compare categorical data. Multiple linear regression analysis was used to evaluate factors associated with the rate of seasonal IOP fluctuation using age, gender, follow-up period, family history of glaucoma, types of glaucoma, laterality, number of glaucoma medications, types of glaucoma medications, MD at first HFA, MD slope, baseline RNFL, RNFL thinning rate, corrected visual acuity, disc haemorrhage, central corneal thickness, and spherical equivalent. Gender was coded as 1 = male and 0 = female. Laterality was coded as 1 = right and 0 = left. Types of glaucoma were coded as 1 = POAG and 0 = NTG. A forward and backward stepwise selection method was used. In order to accurately assess the significant associated factors obtained from the multiple linear regression analysis, an analysis of covariance (ANCOVA) was used to eliminate the effects of confounding factors. Statistical analyses were performed using the statistical program R, version 4.0.0 (available in the public domain http://r-project.org). A P value < 0.05 was considered as statistically significant.
Results
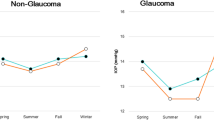
This study enroled 204 eyes from 204 patients with POAG (130 males, 63.3 ± 11.4 years old at last HFA). Characteristics of study participants are shown in Table 1. Eyes with NTG were also included (162 eyes, 79.4%). The mean follow-up period was 140.0 ± 66.9 (25–367) months. The POAG group used a greater number of eye drops than the NTG group (2.0 ± 1.2 vs. 1.5 ± 0.8, P = 0.047). The seasonal IOPs in these groups of glaucoma patients are shown in Table 2. The mean winter IOP of all 204 eyes was significantly higher than the mean summer IOP (13.2 ± 2.7 vs. 12.0 ± 2.3 mmHg, P < 0.001). The winter IOP in the POAG group was higher than in the NTG group (15.9 ± 3.1 vs. 12.5 ± 2.1 mmHg, P < 0.001); similarly, the summer IOP in the POAG group was also higher than in the NTG group (13.9 ± 2.4 vs. 11.5 ± 2.0 mmHg, P < 0.001). The rate of seasonal IOP fluctuation was 13.1 ± 12.9% in the POAG group and 8.1 ± 12.0% in the NTG group (P = 0.002).
Multiple linear regression analysis was used to explore potential variables that might influence the rate of seasonal IOP fluctuation. The MD slope values were not available for three eyes, which were excluded from this analysis. The regression equation as a whole was found to be significant [F (7, 193) = 3.81, P < 0.001], with an R2 of 0.12 (Table 3). Type of glaucoma, family history of glaucoma, and timolol and carteolol use were chosen as the significant associated factors [β = 5.29 (95% CI: 1.80–8.79), P = 0.013; β = −6.27 (95% CI: −2.45 to −10.09), P = 0.007; β = 4.94 (95% CI: 1.21–8.68), P = 0.030; and β = 4.51 (95% CI: 0.87–8.15), P = 0.042, respectively]. The distributions of seasonal IOP fluctuations across study groups are shown using boxplots in Fig. 2. As mentioned above, POAG eyes exhibited a higher rate of seasonal IOP fluctuation than NTG eyes (Fig. 2A and Table 2). On the other hand, there was no significant difference in the rate of IOP fluctuation when the groups were categorized based on family history of glaucoma (P = 0.322, Fig. 2B), nor was any significant difference observed based on timolol (P = 0.159, Fig. 2C) or carteolol (P = 0.114, Fig. 2D) use.
A Boxplot compares the means of seasonal IOP fluctuation rate between POAG and NTG eyes (n = 42 POAG eyes, n = 162 NTG eyes, P = 0.002). B Boxplots showing the % fluctuation rate in the two groups based on the presence of absence of a family history of glaucoma (n = 33 Yes, n = 171 No; P = 0.322). C, D Boxplots showing the % fluctuation rate in those using β-blockers (timolol or carteolol) or not (n = 39 timolol, n = 165 not used, P = 0.159; n = 42 carteolol, n = 162 not used, P = 0.114).
Our results demonstrated that the type of glaucoma was significantly associated with the rate of seasonal IOP fluctuation, and the mean IOP across all seasons in the 42 POAG eyes was higher than that in the 162 NTG eyes. We subsequently used a one-way ANCOVA to verify whether the association between the type of glaucoma and the seasonal IOP fluctuation was due to differences in the IOP values or other factors, such as pathophysiological differences between POAG and NTG. In this analysis, the rate of seasonal IOP fluctuation was set as the dependent variable, glaucoma type was set as the independent variable, and the mean IOP across all seasons was set as the covariate. As a result, the mean IOP across all seasons was not significantly related to the rate of seasonal IOP fluctuation [F (1, 202) = 0.178, P = 0.674]. Glaucoma type, however, did show significant differences in terms of the rate of seasonal IOP fluctuation after eliminating the effect of the mean IOP across all seasons [F (1, 202) = 5.486, P = 0.020].
Discussion
In this study, seasonal IOP fluctuation in POAG eyes was evaluated. We found that winter IOP was significantly higher than summer IOP, which was consistent with previous studies [9,10,11,12,13]. Multiple linear regression analysis demonstrated that the type of glaucoma, a family history of glaucoma, and timolol and carteolol use were independent factors associated with the rate of seasonal IOP fluctuation. The use of glaucoma eye drops had no impact on the fluctuations.
We demonstrated seasonal IOP fluctuations in glaucoma patients living in Tokyo, Japan, where drastic temperatures changes occur seasonally. According to the Japan Meteorological Agency, the maximum monthly average temperature was 27.1 degrees in August and the minimum monthly average temperature was 5.6 degrees in January (Fig. 1). Previous studies that a observed significant seasonal IOP fluctuation were reported from areas with large annual temperature changes [9,10,11,12,13]. Cheng et al. [15]. suggested that changes in air temperature could affect IOP fluctuations. Given these facts, the unique climate of Japan, with its drastic change in temperature, was likely to contribute to our results.
Multiple linear regression analysis demonstrated that, compared to NTG, the diagnosis of POAG was positively associated with the seasonal IOP fluctuation. It was unclear, however, from this finding alone whether the difference in the IOP fluctuation between the POAG and NTG groups was due to a difference in IOP itself or other pathophysiological factors that differ between these two types of glaucoma. Therefore, we performed an additional analysis using an ANCOVA and concluded that some factors other than mean IOP may have affected the IOP fluctuations in these groups. Whereas IOP is the predominant causative risk factor in POAG, other factors are hypothesized to contribute to NTG [17]. These include the disruption of ocular blood flow caused by vascular dysregulation [17] and damage to the optic nerve caused by differences between IOP and intracranial pressure (trans-laminar pressure gradient) [18]. In addition, other studies have demonstrated that NTG is often accompanied by systemic diseases, including Flammer syndrome [19], migraine [20], and Alzheimer’s disease [21], which are not associated with POAG. We speculated that the differences in pathological background between POAG and NTG might contribute to the magnitude of seasonal IOP fluctuations.
Glaucoma eye drops have been reported to reduce IOP fluctuations, such as diurnal IOP fluctuations [22, 23]. Gardiner et al. [11]. suggested that glaucoma eye drops might attenuate seasonal IOP fluctuations. However, which type of eye drop affects seasonal fluctuations has not yet been elucidated. This study indicated that the use of certain β-blockers, specifically timolol and carteolol, might be associated with the seasonal IOP fluctuation. Glaucoma patients who used these eye drops had greater rates of seasonal IOP fluctuation than those who did not, which was inconsistent with previous reports suggesting that eye drops may reduce IOP fluctuations. We interpreted this finding in two ways, as follows: first, monotherapy might be changed to combination therapy in cases of high seasonal IOP fluctuation, especially in cases with high winter IOP. In fact, when comparing the two groups based on β-blocker use, the winter IOP was significantly higher in patients who used β-blockers than in those who did not (14.0 ± 2.8 vs. 12.6 ± 2.5 mmHg, P < 0.001). Timolol and carteolol are the two eye drops that are most commonly chosen in Japan when switching from mono- to combination therapy. Second, the IOP-lowering effects of β-blockers may be weakened in the winter, as Hata et al. [24]. demonstrated increased activity of the sympathetic nervous system as a result of cold exposure in the winter. Also, Cui et al. [25]. suggested that muscular sympathetic nervous system activity varies along with the seasons, with peak levels in the winter. It is unclear to what extent cold stimuli in the winter months contribute to aqueous humour production via sympathetic nervous system activation; however, assuming that is the case, that would partially explain the hypothesis that the efficacy of timolol and carteolol in regulating IOP varies with the seasons. However, to the best of our knowledge, there are no reports of seasonal changes in the effects of β-blockers. As for the association between β-blocker use and seasonal IOP fluctuations, no causal relationships could be elucidated in this study.
Finally, the multiple linear regression analysis showed that a family history of glaucoma was significantly associated with the rate of seasonal IOP fluctuation, with familial glaucoma having a lower rate than sporadic glaucoma. Mabuchi et al. [26]. investigated genetic variants associated with the onset and progression of POAG and demonstrated an association between non-IOP-related genetic variants and a family history of glaucoma. This finding indicated that the genetic determinants differed between familial and sporadic glaucoma, which could explain the association between the IOP fluctuation and family history. In addition, there is a possibility that glaucoma patient with family history could be more motivated and adherent to their medications than sporadic glaucoma patient. Adherence to glaucoma treatment might influence our results.
This study had the following limitations. First, it is possible that this study did not cover all the factors that could influence seasonal IOP fluctuations, including systemic disease and lifestyle behaviour. Cheung et al. [27]. suggested that diabetes and smoking were factors that influenced long-term IOP fluctuations, and Srinvasan et al. [28] showed that diurnal IOP fluctuations were affected by lens thickness and the cup-to-disc ratio. These parameters were not measured in our study, so we cannot confirm or refute these influences. Second, we excluded POAG eyes that had undergone laser and/or surgical treatment. It should be noted that our findings are limited to POAG patients with good outcomes that were successfully managed with eye drops only. Third, although a family history of glaucoma and β-blocker use were selected as factors associated with the seasonal IOP fluctuation by the multiple linear regression analysis, there was no significant difference in the rate of seasonal fluctuation between the two groups based on each factor (boxplots are shown in Figs. 2B, 2C, and 2D). There are several potential reasons to explain these inconsistent results, including the small sample size and alpha error due to the inclusion of many nominal scale variables in the multiple linear regression analysis. In any case, it remains debatable whether these two factors could be accepted as those contributing to IOP fluctuations.
In conclusion, POAG eyes showed significant seasonal IOP fluctuations; winter IOP was higher than summer IOP. POAG eyes had a larger seasonal IOP fluctuation compared to NTG eyes. The differences in disease pathogenesis between the two types of glaucoma might have an impact on the dynamics of the aqueous humour and may contribute to seasonal IOP fluctuations. Familial glaucoma exhibited a lower rate of seasonal IOP fluctuation than sporadic glaucoma, which might be derived from differences in the genetic characteristics related to glaucoma development.
Summary
What was known before
-
Seasonal IOP fluctuations are known to occur in glaucoma eyes, as well as in normal eyes and ocular hypertension. Winter IOP is higher than summer IOP. Although it has been suggested that the seasonal fluctuation is affected by air temperature and sunlight exposure, other associated factors have not been examined.
What this study adds
-
Primary open-angle glaucoma was more prone to seasonal IOP fluctuations than normal tension glaucoma. The differences in underlying pathology between the two glaucoma types, not baseline IOP, could influence the result. Furthermore, family history of glaucoma and beta-blocker might be associated with seasonal IOP fluctuations.
References
Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–51.
Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90.
Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol (Chic, Ill: 1960). 2002;120:1268–79.
Kim JH, Caprioli J. Intraocular pressure fluctuation: is it important? J Ophthalmic Vis Res. 2018;13:170–4.
Itoh Y, Nakamoto K, Horiguchi H, Ogawa S, Noro T, Sato M, et al. Twenty-four-hour variation of intraocular pressure in primary open-angle glaucoma treated with triple eye drops. J Ophthalmol. 2017;2017:4398494–4398494.
Wang NL, Friedman DS, Zhou Q, Guo L, Zhu D, Peng Y, et al. A population-based assessment of 24-hour intraocular pressure among subjects with primary open-angle glaucoma: the handan eye study. Investigative Ophthalmol Vis Sci. 2011;52:7817–21.
Bengtsson B, Leske MC, Hyman L, Heijl A. Fluctuation of intraocular pressure and glaucoma progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:205–9.
Leidl MC, Choi CJ, Syed ZA, Melki SA. Intraocular pressure fluctuation and glaucoma progression: what do we know? Br J Ophthalmol. 2014;98:1315–9.
Qureshi IA, Xi XR, Lu HJ, Wu XD, Huang YB, Shiarkar E. Effect of seasons upon intraocular pressure in healthy population of China. Korean J Ophthalmol: KJO. 1996;10:29–33.
Qureshi IA, Xiao RX, Yang BH, Zhang J, Xiang DW, Hui JL. Seasonal and diurnal variations of ocular pressure in ocular hypertensive subjects in Pakistan. Singap Med J. 1999;40:345–8.
Gardiner SK, Demirel S, Gordon MO, Kass MA. Seasonal changes in visual field sensitivity and intraocular pressure in the ocular hypertension treatment study. Ophthalmology. 2013;120:724–30.
Ayaki M, Negishi K, Yuki K, Kawashima M, Uchino M, Tsubota K. Tear break-up time and seasonal variation in intraocular pressure in a Japanese Population. Diagnostics. 2020;10:124.
Mansouri K, Gillmann K, Rao HL, Weinreb RN Weekly and seasonal changes of intraocular pressure measured with an implanted intraocular telemetry sensor. Br J Ophthalmol. 2020. https://bjo.bmj.com/content/early/2020/06/04/bjophthalmol-2020-315970.info [Epub ahead of print].
Terauchi R, Ogawa S, Noro T, Ito K, Kato T, Tatemichi M et al. Seasonal fluctuation in intraocular pressure and retinal nerve fiber layer thinning in primary open-angle glaucoma. Ophthalmology Glaucoma 2020. https://www.sciencedirect.com/science/article/pii/S2589419620303057 [Epub ahead of print].
Cheng J, Xiao M, Xu H, Fang S, Chen X, Kong X, et al. Seasonal changes of 24-hour intraocular pressure rhythm in healthy Shanghai population. Medicine. 2016;95:e4453.
Iwase A, Suzuki Y, Araie M, Yamamoto T, Abe H, Shirato S, et al. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology. 2004;111:1641–8.
Trivli A, Koliarakis I, Terzidou C, Goulielmos GN, Siganos CS, Spandidos DA, et al. Normal-tension glaucoma: pathogenesis and genetics. Exp Therapeutic Med. 2019;17:563–74.
Ren R, Jonas JB, Tian G, Zhen Y, Ma K, Li S, et al. Cerebrospinal fluid pressure in glaucoma: a prospective study. Ophthalmology. 2010;117:259–66.
Flammer J, Konieczka K, Flammer AJ. The primary vascular dysregulation syndrome: implications for eye diseases. EPMA J. 2013;4:14.
Cursiefen C, Wisse M, Cursiefen S, Jünemann A, Martus P, Korth M. Migraine and tension headache in high-pressure and normal-pressure glaucoma. Am J Ophthalmol. 2000;129:102–4.
Sugiyama T, Utsunomiya K, Ota H, Ogura Y, Narabayashi I, Ikeda T. Comparative study of cerebral blood flow in patients with normal-tension glaucoma and control subjects. Am J Ophthalmol. 2006;141:394–6.
David R, Zangwill L, Briscoe D, Dagan M, Yagev R, Yassur Y. Diurnal intraocular pressure variations: an analysis of 690 diurnal curves. Br J Ophthalmol. 1992;76:280–3.
Varma R, Hwang LJ, Grunden JW, Bean GW. Using diurnal intraocular pressure fluctuation to assess the efficacy of fixed-combination latanoprost/timolol versus latanoprost or timolol monotherapy. Br J Ophthalmol. 2010;94:80–4.
Hata T, Ogihara T, Maruyama A, Mikami H, Nakamaru M, Naka T, et al. The seasonal variation of blood pressure in patients with essential hypertension. Clin Exp hypertension Part A Theory Pract. 1982;4:341–54.
Cui J, Muller MD, Blaha C, Kunselman AR, Sinoway LI. Seasonal variation in muscle sympathetic nerve activity. Physiological Reports. 2015;3:e12492.
Mabuchi F, Mabuchi N, Sakurada Y, Yoneyama S, Kashiwagi K, Iijima H, et al. Genetic variants associated with the onset and progression of primary open-angle glaucoma. Am J Ophthalmol. 2020;215:135–40.
Cheung CY, Li SL, Chan N, Wong MO, Chan PP, Lai I, et al. Factors associated with long-term intraocular pressure fluctuation in primary angle closure disease: the CUHK PACG Longitudinal (CUPAL) Study. J Glaucoma. 2018;27:703–10.
Srinivasan S, Choudhari NS, Baskaran M, George RJ, Shantha B, Vijaya L. Diurnal intraocular pressure fluctuation and its risk factors in angle-closure and open-angle glaucoma. Eye. 2016;30:362–8.
Acknowledgements
This research was supported by a Research Grant commissioned by Japan Society of Ningen Dock (grant number: 2018-7, to RT); JSPS KAKENHI (grant number: 20K18396, to SO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Terauchi, R., Ogawa, S., Sotozono, A. et al. Seasonal fluctuation in intraocular pressure and its associated factors in primary open-angle glaucoma. Eye 35, 3325–3332 (2021). https://doi.org/10.1038/s41433-021-01403-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01403-6
This article is cited by
-
The association between intraocular pressure dynamics during dark-room prone testing and intraocular pressure over a relatively long-term follow-up period in primary open-glaucoma patients
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
-
Impacts of heatwaves and cold spells on glaucoma in rural China: a national cross-sectional study
Environmental Science and Pollution Research (2023)
-
Low ambient temperature and temperature drop as novel risk factors of acute glaucoma: a case-crossover study
Environmental Science and Pollution Research (2023)
-
Association Between Diabetes, Diabetic Retinopathy, and Glaucoma
Current Diabetes Reports (2021)